Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines for Chiari Malformation: Surgical Interventions
Download PDF Neurosurgery, 2023
Sponsored by: Congress of Neurological Surgeons (CNS) and the Section on Pediatrics
Endorsed by: The Congress of Neurological Surgeons (CNS), American Association of Neurological Surgeons (AANS) and the Bobby Jones Chiari & Syringomyelia Foundation (Bobby Jones CSF)
Authors:
Jogi V. Pattisapu MD FAAP FACS FAANS1, Laurie L Ackerman, MD2, Libby Kosnik Infinger, MD, MPH3, Cormac O. Maher, MD, FAAP, FACS, FAANS4, Carolyn Quinsey, MD5, Brandon G. Rocque, MD, MS6, Howard Silberstein, MD7, Eric M. Jackson, MD8, Sarah Jernigan, MD, MPH9, Toba Niazi, MD10, Rabia Qaiser, MD11, Jeffrey S. Raskin MS MD12, Shobhan Vachhrajani MD, PhD, FRCSC13, David F. Bauer, MD, MPH14
Departmental and institutional affiliations:
- Pediatric Neurosurgery, University of Central Florida College of Medicine, Orlando FL
- Department of Neurological Surgery, Indiana University Health, Indianapolis, IN
- Department of Neurosurgery, Medical University of South Carolina (MUSC), Charleston, SC
- Department of Neurosurgery, Stanford Medicine, Palo Alto, CA
- Department of Neurosurgery, University of North Carolina Chapel Hill, Chapel Hill, NC
- Division of Pediatric Neurosurgery, Department of Neurosurgery, University of Alabama at Birmingham, Birmingham, AL
- Department of Neurosurgery, University of Rochester School of Medicine and Dentistry, Rochester, NY
- Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD
- Carolina Neurosurgery & Spine Associates, Charlotte, NC
- Department of Neurological Surgery, Nicklaus Children's Hospital, Miami, FL
- Department of Neurological Surgery, Indiana University School of Medicine, Indianapolis, IN
- Department of Neurological Surgery, Northwestern University Feinberg School of Medicine, Chicago, IL
- Department of Pediatrics, Wright State University Boonshoft School of Medicine, Dayton, OH
- Department of Neurosurgery, Baylor College of Medicine, Division of Pediatric Neurosurgery, Texas Children’s Hospital, Houston, TX
Corresponding Author contact information:
No part of this article has been published or submitted for publication elsewhere.
Keywords:
Abbreviations: Chiari malformation type I, CIM; Chicago Chiari Outcome Scale, CCOS; intraoperative brainstem auditory evoked potentials, BAEPs; intraoperative neuromonitoring, IONM; posterior fossa decompression, PFD; posterior fossa decompression with duraplasty, PFDD; somatosensory evoked potentials, SSEPs
ABSTRACT
Background: Chiari malformation type I (CIM) diagnoses have increased in recent years. Patients may develop delayed symptoms or syringomyelia requiring surgical intervention, while mild symptoms can be managed conservatively. Controversy regarding the best operative management prompted a review of literature to offer guidance on surgical interventions.
Objective: This evidence-based clinical practice guidelines assessed literature to determine: 1) whether posterior fossa decompression or posterior fossa decompression with duraplasty is more effective in preoperative symptom resolution; 2) if there is benefit from cerebellar tonsillar resection/reduction; 3) the role of intraoperative neuromonitoring; 4) in patients with a syrinx, how long should a syrinx be observed for improvement before additional surgery is performed; and 5) what is the optimal duration of follow-up care after preoperative symptom resolution.
Methods: A systematic review was performed using the National Library of Medicine/PubMed and Embase databases for studies on CIM in children and adults. The most appropriate surgical interventions, the use of neuromonitoring, and clinical improvement during follow-up were reviewed for studies published between 1946 and January 23, 2021.
Results: A total of 80 studies met inclusion criteria and there was no Class I evidence in the literature. Posterior fossa decompression with or without duraplasty or cerebellar tonsil reduction all appeared to show some benefit for symptom relief and syrinx reduction. There was insufficient evidence to determine if duraplasty or cerebellar tonsil reduction was needed for specific patient groups. There was no strong correlation between symptom relief and syringomyelia resolution. Many surgeons follow patients for 6 to 12 months before considering reoperation for persistent syringomyelia. No benefit or harm was seen with the use of neuromonitoring.
Conclusion: This evidence-based clinical guidelines for the treatment of CIM provide 1 Class II and 4 Class III recommendations. In patients with CIM with or without syringomyelia, treatment options include bone decompression with or without duraplasty or cerebellar tonsil reduction. Improved syrinx resolution may potentially be seen with dural patch grafting. Symptom resolution and syrinx resolution did not correlate directly. Reoperation for a persistent syrinx was potentially beneficial if the syrinx had not improved 6 to 12 months after the initial operation.
RECOMMENDATIONS
3-1. In patients with CIM (with or without syrinx), what type of surgery most often improves preoperative symptoms: posterior fossa decompression (PFD) or posterior fossa decompression with duraplasty (PFDD)?
Recommendation: In patients with symptomatic CM1 malformation (with or without syrinx), either posterior fossa decompression (PFD) or posterior fossa decompression with duraplasty (PFDD) may be utilized as a first line treatment to improve pre-operative symptoms.
Strength of recommendation: Grade C
Class III evidence
3-2. In patients with CIM, is cerebellar tonsil reduction beneficial?
Recommendation: In patients undergoing posterior fossa decompression surgery for treatment of CIM and syrinx, surgeons may perform resection or reduction of cerebellar tonsil tissue to improve syrinx and/or symptoms.
Strength of recommendation: Grade C
Class III evidence
3-3. Is there a role for intraoperative neuromonitoring in patients undergoing decompression for Chiari I malformation?
Recommendation: Intraoperative neuromonitoring in patients undergoing decompression for CIM may be used during surgery.
Strength of recommendation: Grade C
Class III evidence
3-4. In patients with CIM and syrinx, how long should you wait to evaluate for syrinx reduction prior to performing additional surgery?
Recommendation: Surgeons may perform additional neurosurgical intervention 6 to 12 months following surgical treatment of CIM with syringomyelia in patients that have not demonstrated radiographic improvement.
Strength of recommendation: Grade B
Class II evidence
3-5. In surgically treated patients with CIM who have improved, is long term follow up needed?
Recommendation: Patients undergoing surgery for CIM who experience symptom resolution may be monitored for symptom or imaging changes.
Strength of recommendation: Grade C
Class III evidence
INTRODUCTION
Goals and Rationale
Approximately 0.24% to 2.6% of the population,1–5 including children and adults, is affected by Chiari malformation type I (CIM). CIM is defined as descent of the cerebellar tonsils ≥3 to 5 mm below the foramen magnum. Not all patients are symptomatic, and there are various ways to diagnose and treat patients with CIM. CIM may cause syringomyelia, and some patients with CIM may have craniocervical instability requiring decompression and/or fusion of the craniocervical junction. Symptoms result from blockage of the flow of cerebrospinal fluid (CSF) or from compression of the brainstem or cranial nerves. In some cases, other neurologic or orthopedic conditions are also present, which can make diagnosis or management challenging for clinicians and their patients.
This guideline was developed to determine the most appropriate diagnostic and surgical management in adult and pediatric patients with CIM (with or without syrinx), based on current literature. Procedures likely to improve preoperative symptoms or clinical findings were reviewed, as well as ancillary interventions, including cerebellar tonsil reduction or intraoperative neuromonitoring (IONM). Timing for reimaging or repeat intervention is also included to inform long term assessment and follow-up.
This guideline is intended to help improve patient care by outlining appropriate information gathering and decision-making processes involved in the treatment of patients with CIM. Surgical care is provided by different clinicians, and they were created as an educational tool to navigate through a series of diagnostic and treatment decisions to this condition.
The ultimate surgical judgment regarding any specific procedure or treatment must be made in view of the patient’s presenting circumstances. Therefore, the guidelines should not be construed as all-inclusive or excluding any reasonable methods directed towards obtaining the same results.
METHODOLOGY
The guidelines task force initiated a systematic review of the evidence-based literature relevant to the treatment of patients with CIM. Through objective evaluation of the evidence and transparency in the process of making recommendations, these evidence-based clinical practice guidelines were developed for the diagnosis and treatment of patients with CIM, mainly as an educational resource to assist practitioners during clinical decision-making processes. Additional information about the methods used in this systematic review is provided below.
Literature Search
Task force members identified search terms/parameter and a medical librarian implemented the literature search, consistent with the literature search protocol (see Appendix I), using the National Library of Medicine/PubMed database and Embase for the period from 1946 to January 23, 2021 using the search strategies provided in Appendix I.
Inclusion/Exclusion Criteria
Articles were retrieved and included only if they met specific inclusion/exclusion criteria. To reduce bias, these criteria were specified before conducting the literature searches.
Articles that do not meet the following criteria were, for the purposes of this evidence-based clinical practice guideline, excluded. To be included as evidence in the guideline, an article had to be a report of a study that:
- Investigated patients with CIM;
- Studies that enrolled ≥80% of CIM (studies with mixed patient populations were included if results were separately reported for each group/patient population);
- Was a full article report of a clinical study;
- Was not a medical records review, meeting abstract, historical article, editorial, letter, or a commentary;
- Appeared in a peer-reviewed publication or a registry report;
- Enrolled a minimum of 10 patients;
- Was of humans;
- Was published between 1946 and January 23, 2021;
- Quantitatively presented results;
- Was not an in vitro study;
- Was not a biomechanical study;
- Was not performed on cadavers;
- Was published in English;
- Was not a systematic review, meta-analysis, or guideline developed by others1
Systematic reviews or meta-analyses conducted by others, or guidelines developed by others were not included as evidence to support this review due to the differences in article inclusion/exclusion criteria compared to those criteria specified by the Guidelines Task Force. Although these articles were not included as evidence to support the review, they were recalled for full-text discussion and conduct manual searches of the bibliographies.
Assessment for Risk of Bias
The methodological quality of randomized controlled trials and the risk of bias were assessed using the following 6 criteria:
1. Sequence generation (Was the allocation sequence adequately generated?)
2. Allocation concealment (Was allocation adequately concealed such that it could not be foretold?)
3. Blinding (Were participants, treatment providers and/or outcome assessors blinded to the treatment allocations?)
4. Incomplete reporting of data (Were incomplete outcome data adequately addressed?)
5. Selective reporting of outcomes (Were all the outcomes specified reported?)
6. Other potential threats to validity (Was the randomized controlled trial free of other issues that could put it at a high risk of bias?)
In the case of nonrandomized observational evidence, potential threats to the validity of the data were assessed by examining for:
1. Bias due to selective case choice for study and selective result reporting,
2. Bias due lack or loss of information over time,
3. The biases of the interpreting investigator regarding the study
4. Publication bias regarding positive studies or positive cases
5. Misclassification
6. Survivorship bias
7. Publication bias
8. Recognition that in data collected in a retrospective or prospective manner correlation does not imply causation
9. Election bias
10. Attrition bias
11. Bias of change in methods over time
12. Ascertainment bias
Rating Quality of Evidence
The quality of evidence was rated using an evidence hierarchy for each of 4 different study types; therapeutic, prognostic, diagnostic, and decision modeling. These hierarchies are shown in Appendix II: Rating Evidence Quality. Additional information regarding the hierarchy classification of evidence can be located here: https://www.cns.org/guidelines/guideline-procedures-policies/guideline-development-methodology.
Revision Plans
In accordance with the Institute of Medicine’s standards for developing clinical practice guidelines and criteria specified by the National Guideline Clearinghouse, the task force will monitor related publications following the release of this document and will revise the entire document and/or specific sections “if new evidence shows that a recommended intervention causes previously unknown substantial harm; that a new intervention is significantly superior to a previously recommended intervention from an efficacy or harms perspective; or that a recommendation can be applied to new populations.”6 In addition, the task force will confirm within five years from the date of publication that the content reflects current clinical practice and the available technologies for the evaluation and treatment for patients with CIM.
RESULTS
The literature search yielded 760 abstracts. Task force members reviewed all abstracts yielded from the literature search and identified articles for full-text review and extraction to address the clinical questions, in accordance with the literature search protocol (Appendix I). Task force members identified the best research evidence available to answer the targeted clinical questions. When class I, II, and or III literature was available to answer specific questions, the task force did not review class IV studies.
Of the 760 abstracts, 680 did not meet inclusion criteria or were off-topic and the remaining 80 were selected for systematic review (Appendix III).
DISCUSSION
In general, extradural treatment of CIM consists of suboccipital decompression, C1 laminectomy, with or without resection of occipital cervical ligament with possible scoring of the dura. Intradural or “duraplasty” consists of the standard suboccipital decompression with C1 laminectomy, in addition to opening of the dura with potential reduction of the cerebellar tonsisl with cautery or resection, with possible exploration of fourth ventricle outflow, and finally duraplasty to expand the dura. Duraplasty can consist of either autograft or allograft.
Question 3-1. In patients with CIM (with or without syrinx), what type of surgery most often improves preoperative symptoms (syrinx, headache, etc): posterior fossa decompression (PFD) or posterior fossa decompression with duraplasty (PFDD)?
Recommendations: In patients with symptomatic CIM malformation (with or without syrinx), either PFD or PFDD may be used as a first-line treatment to improve preoperative symptoms.
Strength of recommendation: Grade C
Most studies (n = 17) met the criteria for class III evidence with 1 study meeting criteria for class II evidence. No studies met criteria for class I evidence.
An article by the Park-Reeves Syringomyelia consortium7 was published after the literature search was completed for this guideline and therefore was not included in the initial systematic review or guideline recommendations. Nevertheless, the results are pertinent and are included in this discussion. This study compared outcomes in 117 patients that had PFD to 575 patients that had PFDD. All patients had tonsil position ≥5 mm below the foramen magnum and a syrinx diameter >3 mm. The mean postoperative follow-up interval was 2.7 years, with a minimum of 1 year. PFDD was strongly associated with improved outcomes with respect to headache (89.6% vs 80.8%) and a significantly greater reduction in syrinx diameter (47.7% vs 26.9%), and had a higher rate of pseudomeningocele (7.7% vs 2.6%).
Class II Evidence
One class II study was identified. Pisapia et al8 performed a retrospective cohort study of 189 patients, comparing intradural and extradural PFDs. The surgical technique was selected by the surgeon and the extradural approach was associated with a high rate of occipital headache resolution compared with the intradural method. Otherwise, there was no difference in rates of symptom resolution or syrinx size based on these 2 surgical approaches. The intradural group had statistically significant more pseudomeningocele formation (18% vs 0%) and chemical meningitis (10% vs 0%). However, there was no difference in the need for reoperation in these patients.
Class III Evidence
Most authors included the degree of symptom resolutions, syrinx resolution, need for reoperation, and complication rates.
Symptom Resolution
Multiple authors reported an improvement in symptom improvement (such as headaches, neck pain, or visual complaints) in the PFDD over the PFD approach.9–11 Gurbuz et al9 found 93% improved with PFDD versus 50% with PFD if symptoms were <36 months’ duration. Gallo et al10 also reported better outcomes in the PFDD group, although the changes were not statistically significant. Yilmaz et al11 also reported a 56.3% recovery rate and 89.6% improvement with PFDD versus a 51.9% recovery rate and 79.1% improvement rate with PFD. Interestingly, Pandey et al12 and Shimoji et al13 found no significant differences between the groups; however, Pandey et al had several missing data points. Jiang et al14 found no significant difference in the Chicago Chiari Outcome Scale (CCOS) between PFD and PFDD in their small group. Butensky et al15 performed a large retrospective study and found no difference in symptom resolution, and other similar studies reported impressive symptom improvement rates by PFDD alone.16,17 A small, retrospective study found that patients with PFDD had a greater range of cervical motion compared with patients with PFD.18
Syrinx Resolution
Most articles identified improved syrinx resolution in patients undergoing PFDD versus PFD,9–12,15,19,20 although 1 series reported a slight advantage in PFD.13 Others found no difference with relatively small comparison groups.14,21 Gurbuz et al9 noted a syrinx regression rate of 92.3% with PFDD versus 12.5% with PFD. Gallo et al10 reported 92% improvement with PFDD versus 80% with PFD. Butensky et al15 found that 93% of syrinxes improved after PFDD versus 62% of the small number receiving PFD alone.15 Yilmaz et al11 reported 91.1% improvement with PFDD versus 84.2% with PFD alone. Interestingly Pandey et al12 noted the best results with PFD with dural splitting (86%) versus PFDD (60%) and Chotai et al17 documented a mean time to 50% resolution of syrinx in PFDD of 8 months. Although Jiang et al14 had a slightly higher rate of syrinx resolution after PFDD than PFD, the patient numbers in that series were too small to draw any conclusions.
Complications
Reported complications such as pseudomeningocele or CSF leak were more frequently cited with PFDD versus PFD.9–11,13–15,17,21,22 Gurbuz et al9 reported a 28.6% complication rate in PFDD versus 5.6% in PFD. Although not statistically significant, CSF leak, infection, and pseudomeningocele were more frequently seen in PFDD. Similarly, Jiang et al14 found that a higher percentage of patients with PFDD experienced CSF complications, while Shimoji et al13 reported 1 CSF leak and 2 pseudomeningocles in 11 PFDD operations. There were 10 complications in 85 cases reported by Chotai et al,17 although details were not readily available. In this series with PFD and PFDD, 3 patients had aseptic meningitis, 4 patients developed CSF leaks, 1 with hydrocephalus, and 2 had persistent headaches. Klekamp et al23 reported a complication rate of 21.8% in 371 patients undergoing PFDD, with CSF fistula in 5.5% of cases, aseptic meningitis in 4.3%, and hydrocephalus in 3.1%. This article also noted that severe arachnoid scarring tended to portend higher complication and reoperation rates. Yilmaz et al11 also reported a statistically significant increase in complications (wound infection, CSF leak, etc) in PFDD compared with PFD.
Need for Reoperation
Reoperations occurred more frequently in the PFD group, but this was not statistically significant.9,10,24 Gurbuz et al9 reported a 22.2% reoperation rate in PFD versus 14.3% in PFDD. Gallo et al10 reported 15% in his series and 6% in the PFDD group. Conversely, Shimoji et al13 noted 1 reoperation in 11 cases of PFDD group, and Klekamp23 reported 45 reoperations in his series of 371 PFDDs and noted severe arachnoid scarring as a reason for reoperation. Yilmaz et al11 reported a 3.6% rate of reoperation for CSF fistula in the PFDD group versus a 9.5% reoperation rate for inadequate decompression in the PFD group. The literature suggested mixed results with symptom or syrinx resolution, complications, and need for reoperations. Since there was no class I evidence to support the use of either PFD or PFDD, either approach may be used as a first-line treatment for CIM.
Question 3-2. In patients with CIM, is cerebellar tonsil reduction beneficial?
Recommendation: In patients undergoing PFD surgery for treatment of CIM and syrinx, surgeons may perform resection or reduction of cerebellar tonsil tissue to improve syrinx and/or symptoms.
Strength of recommendation: Grade C
Several retrospective studies and a single prospective study assessed outcomes after CIM decompression with or without manipulation of the cerebellar tonsils. All studies included for review considered syrinx resolution as the primary outcome, therefore an independent assessment of clinical symptom resolution was not possible. Accordingly, this recommendation included syringomyelia reduction as the outcome. Some studies reported better syrinx resolution with tonsil manipulation, while other studies reported higher complication rates with tonsil manipulation. Overall, there was not an obvious benefit or harm from tonsil manipulation apparent across studies.
Class III Evidence
There were 10 studies that directly compared CIM PFDD with and without manipulation of the cerebellar tonsils (including tonsil dissection, resection, cauterization, or some combination thereof). In 6 studies, there was no difference in outcomes with either option.25–30 In 3 studies,17,31,32 resolution of syringomyelia was more likely when tonsil manipulation was performed. Conversely, 1 study suggested patients who did not undergo tonsil manipulation had a higher likelihood of resolution of syringomyelia.33
Seven studies17,25,28,29,31–33 reported no difference in complication rates, regardless of tonsillar manipulation, while 3 studies reported higher complication rates with tonsil manipulation.26,27,30 In the single prospective and nonrandomized study, no difference in outcomes or complications was reported.29
Question 3-3. Is there a role for IONM in patients undergoing decompression for CIM?
Recommendation: IONM in patients undergoing decompression for CIM may be used during surgery.
Strength of recommendation: Grade C
All studies met the criteria for class III evidence. None met the criteria for class II or I evidence.
Class III Evidence
Anderson et al34 performed a retrospective study to determine if intraoperative brainstem auditory evoked potentials (BAEPs) changes might determine the extent of decompression necessary and if changes occurred during operative positioning. They reported that conduction through the brainstem improves substantially after bony opening, but only marginally after dural opening. The authors suggested both BAEPs and somatosensory evoked potentials (SSEPs) might identify early changes during operative positioning and may help prevent adverse outcomes. In support of these findings, Barzilai et al35 found that IONM can be useful in PFD, particularly during patient positioning. Class III evidence supports the use of IONM, but it is not necessary in patients undergoing decompression of a CIM.
Anderson et al36 went on to perform a IONM prospective study to determine which patients may respond to bony decompression alone. The authors performed a continuous study of intraoperative BAEPs in patients undergoing PFDD and compared conduction at 3 points: 1) at baseline when patient is supine before positioning, 2) immediately after bony decompression and release of the atlanto-occipital membrane, and 3) after dural opening. They concluded that significant improvement in brainstem conduction immediately occurs after bone decompression and division of the atlanto-occipital membrane, rather than dural opening.
Data from Zamel et al37 further support this finding of intraoperative BAEP changes during different surgical stages of CIM repair. They reviewed variations during different aspects of surgery and correlated them with clinical or radiologic findings. In both groups (with or without syringomyelia) the predominant improvement in central conduction occurred during the period of bony decompression without significant additional improvement after the duraplasty.
Another group38 reviewed their experience with IONM during pediatric CIM surgery and did not identify a definite correlation between clinical outcomes or syrinx improvement.
Subtle nonsignificant changes in SSEP/MEPs during suboccipital decompression (without clinical correlation) were reported in adults by Roser et al.39 The authors felt that IONM is not considered a prerequisite for a safe suboccipital decompression when surgery is performed by an experienced team. In support of these findings, Barzilai et al35 found that IONM can be useful in CIM surgery, particularly during patient positioning.
Class III evidence supports the use of IONM, but it is not necessary in patients undergoing decompression of a CIM.
Question 3-4. In patients with CIM and syrinx, how long should you wait to evaluate for syrinx reduction before performing additional surgery?
Recommendation: Surgeons may perform additional neurosurgical intervention for 6 to 12 months after surgical treatment of CIM with syringomyelia in patients that have not demonstrated radiographic improvement.
Strength of recommendation: Grade B
The decision to reoperate on a patient with CIM and syringomyelia will require consideration of multiple factors (such as presenting clinical symptoms/signs or radiographic evidence of syrinx improvement/resolution).
Reported timetables for observing postoperative syrinx improvement by MRI imaging vary widely. While some studies report syrinx improvement as early as 3 to 4 months, most studies suggest follow-up for 6 to 7 months; the remaining studies report further syrinx resolution by 1 year in 80% of cases. Beyond 12 months, patients will likely experience late stability, resolution, or expansion of their syrinx (often without symptom changes).
Numerous retrospective publications that discussed syringomyelia reduction and resolution after neurosurgical intervention were included. The degree of granularity and emphasis on this radiographic outcome varied among these reports.
Hale et al40 reported on time to syrinx resolution comparing PFD and PFDD approaches. A similar study evaluating use of tonsillar coagulation was done by Stanko et al.32 El-Ghandour41 reported a group of patients treated with a variety of approaches: PFD, PFD with fourth ventricle stent, and patients treated with syrinx shunting. Soleman et al42 reported syrinx reduction times (mean 3.4 months) for 21 patients with CIM and syringomyelia treated by syrinx shunting.
The remaining 16 retrospective studies17,32,43–58 reported times for syrinx response to surgical PFD procedures. They did not compare differences in surgical techniques.
Available evidence suggests additional neurosurgical interventions after surgical treatment of patients with CIM with syringomyelia may be needed for patients that have not demonstrated radiographic improvement within 6 to 12 months postsurgery.
Question 3-5. In surgically treated patients with CIM who have clinically improved, is long-term follow-up needed?
Recommendation: Patients undergoing surgery for CIM who experience symptom resolution may be monitored for symptom or imaging changes.
Strength of recommendation: Grade C
Neurosurgeons routinely follow patients with CIM postoperatively at varied scheduled intervals within 12 months, according to their practice routine. Long-term follow-up (follow-up >1 year) varies widely.
Many reports included patients who initially improve after CIM surgery but who require another operative intervention for recurrence of symptoms or a syrinx years later.
Twenty-six publications8,17,51,52,57–78 reported long-term follow-up and clinical status after Chiari decompression surgery. All are retrospective case series, with inconsistent long-term criteria for reoperation or intervention. It is unclear if planned long-term follow-up or an unscheduled return for symptom evaluation yield different reoperation rates or clinical outcomes.
In these retrospective studies, patients were evaluated clinically or for syrinx recurrence, but do not include details for the repeat assessment (ie, routine/scheduled long-term follow-up or return of symptoms). Five studies found significantly elevated reoperation rates (12-50%), mostly occurring after 6 to 12 months.59,62,67,75,76 Nine studies noted a 1.1% to 9% reoperation rate for symptoms or imaging findings, consistent with previous reports,8,17,58,60,64,65,68,71,73 and 8 studies had long-term follow-up without clinical indication for reoperation.61,63,66,70,72,74,77,78 Two studies reported patient deterioration or syrinx progression after 1 year; however, patients did not undergo surgery.51,52
Two studies identified long-term scoliosis progression referred for surgical management; however, it was unclear if the initial neurosurgeon or scoliosis physician performed the evaluations.69,73
Future Directions
This systematic review of the literature highlighted variations in clinical practices. No specific recommendations can be made on the best surgical approach, the need for dural patch grafting, or cerebellar tonsillar resection. There is significant need for prospective studies to evaluate the use of duraplasty and cerebellar tonsil reduction to determine which patients may potentially benefit from these practices. In most instances, symptoms and syrinx improved within 6 to 12 months of a successful operation. Some clinicians follow patients for 6 to 12 months, and other clinicians follow for much longer. Further research into the age of a patient at initial decompression and the optimal duration of follow up care is needed. Recent data7–11 suggest better outcomes using a dural patch graft without increase in complication rates. Further studies are necessary to confirm these results, and more data are expected for the planned update of these guidelines in 5 years.
Future studies and collaborative efforts may offer more insights to improve our management approach. Patient-centered studies evaluating patient-reported outcomes may be helpful to inform future clinical decision making and recommendations. It is imperative to explore these questions to help improve care of our patients with CIM and syringomyelia.
CONCLUSIONS
Mostly class III and some class II evidence informed our recommendations for the treatment of CIM. Decompression with or without duraplasty and with or without cerebellar tonsil reduction seemed to provide symptom relief and syrinx resolution. Recent evidence (published after evidence review) suggests improved outcomes with duraplasty. Benefit of intraoperative monitoring was inconclusive, and it is common to wait 6 to 12 months for symptom or syrinx resolution before considering a repeat operation.
The guidelines offer a review of current evidence in the literature. We anticipate upcoming high-quality studies to develop revision of these guidelines to allow improved care for our patients with CIM.
Conflicts of Interest
All Guideline Task Force members were required to disclose all potential COIs prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Review Committee. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination and participation on the task force. The CNS Guidelines Committee and Guideline Task Force Chair may approve nominations of task force members with possible conflicts and restrict the writing, reviewing, and/or voting privileges of that person to topics that are unrelated to the possible COIs. See Appendix V for a complete list of disclosures.
Disclosure of Funding
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document.
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a physician volunteer task force as an educational tool that reflects the current state of knowledge at the time of completion. Each chapter is designed to provide an accurate review of the subject matter covered. This guideline is disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Acknowledgments
The guidelines task force would like to acknowledge the CNS Guidelines Committee for their contributions throughout the development of the guideline, the AANS/CNS Joint Guidelines Review Committee, as well as the contributions of Kirsten Aquino, contracted project manager for the CNS, Trish Rehring, MPH, Associate Director for Evidence-Based Practice Initiatives for the CNS, and Janet Waters, MLS, BSN, RN, for assistance with the literature searches. The guidelines task force would also like to acknowledge the contributions of Dorothy Poppe, Kaitlyn Esposito, MPH and Mary Poppe, as well as the Bobby Jones Chiari and Syringomyelia Foundation for serving as patient advocates on this guideline task force1. Throughout the review process, the reviewers and authors were blinded from one another. At this time the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Jennifer Sweet, MD, Andrew Carlson, MD, MS, Matthew Reynolds, MD, PhD, Alexandra D. Beier, D.O., FACOS, FAAP, Jonathan Pindrik, MD and Patti Raksin, MD.
1The guideline task force did not include systematic reviews, guidelines, or meta-analyses conducted by others. These documents are developed using different inclusion criteria than those specified in this guideline; therefore, they may include studies that do not meet the inclusion criteria specific to this guideline. In cases where these types of documents’ abstract suggested relevance to the guideline’s recommendations, the task force searched their bibliographies for additional studies.
REFERENCES
1. Aitken LA, Lindan CE, Sidney S, et al. Chiari type I malformation in a pediatric population. Pediatric Neurology. 2009;40(6):449-454.
2. Meadows J, Kraut M, Guarnieri M, Haroun RI, Carson BS. Asymptomatic Chiari Type I malformations identified on magnetic resonance imaging. Journal of neurosurgery. 2000;92(6):920-926.
3. Morris Z, Whiteley WN, Longstreth Jr WT, et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ (Online). 2009;339(7720):547-550.
4. Strahle J, Muraszko KM, Kapurch J, Bapuraj JR, Garton HJ, Maher CO. Chiari malformation type I and syrinx in children undergoing magnetic resonance imaging. Journal of neurosurgery Pediatrics. 2011;8(2):205-213.
5. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. New England Journal of Medicine. 2007;357(18):1821-1828.
6. Ransohoff DF, M. Pignone, and H.C. Sox, . How to decide whether a clinical practice guideline is trustworthy. . JAMA. 2013;309(2):139-140.
7. Akbari SHA, Yahanda AT, Ackerman LL, et al. Complications and outcomes of posterior fossa decompression with duraplasty versus without duraplasty for pediatric patients with Chiari malformation type I and syringomyelia: a study from the Park-Reeves Syringomyelia Research Consortium. Journal of Neurosurgery: Pediatrics. 2022:1-13.
8. Pisapia JM, Merkow MB, Brewington D, et al. External validity of the chiari severity index and outcomes among pediatric chiari I patients treated with intra- or extra-Dural decompression. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2016;33(2):313-320.
9. Gurbuz MS, Karaaslan N, Caliskan T, Unal E, Berkman MZ. Comparison of the surgical results for foramen magnum decompression with and without duraplasty in Chiari malformation type 1. Turkish neurosurgery. 2015;25(3):419-424.
10. Gallo P, Sokol D, Kaliaperumal C, Kandasamy J. Comparison of three different cranio-cervical decompression procedures in children with Chiari malformation type I: does the surgical technique matter? Pediatric neurosurgery. 2017;52(5):289-297.
11. Yilmaz A, Kanat A, Musluman AM, et al. When is duraplasty required in the surgical treatment of Chiari malformation type I based on tonsillar descending grading scale? World neurosurgery. 2011;75(2):307-313.
12. Pandey S, Li L, Wan RH, Gao L, Xu W, Cui DM. A retrospective study on outcomes following posterior fossa decompression with dural splitting surgery in patients with Chiari type I malformation. Clinical neurology and neurosurgery. 2020;196:106035.
13. Shimoji K, Hara T, Ohara Y. Controversies related to pediatric Chiari I malformation. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2019;35(10):1695-1699.
14. Jiang E, Sha S, Yuan X, et al. Comparison of clinical and radiographic outcomes for posterior fossa decompression with and without duraplasty for treatment of pediatric Chiari I malformation: a prospective study. World neurosurgery. 2017;110:e465-e472.
15. Butensky S, Rodgers S, Baron S, Schneider S, Mittler M. Comparison of surgical outcomes in patients with Chiari type I malformation receiving posterior fossa decompression with and without duraplasty. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2020;36(7):1399-1405.
16. Bao CS, Liu L, Wang B, et al. Craniocervical decompression with duraplasty and cerebellar tonsillectomy as treatment for Chiari malformation-I complicated with syringomyelia. Genetics and molecular research : GMR. 2015;14(1):952-960.
17. Chotai S, Chan EW, Ladner TR, et al. Timing of syrinx reduction and stabilization after posterior fossa decompression for pediatric Chiari malformation type I. Journal of neurosurgery Pediatrics. 2020:1-7.
18. Yilmaz A, Urgun K, Aoun SG, et al. Adding expansile duraplasty to posterior fossa decompression may restore cervical range of motion in grade 3 Chiari malformation type 1 patients. World neurosurgery. 2016;98:98-103.
19. Ito K, Yamada M, Horiuchi T, Hongo K. Appropriate surgical procedures for Chiari type 1 malformation and associated syrinx based on radiological characteristics of the craniovertebral junction. Neurosurgical review. 2019;43(2):575-580.
20. Oral S, Yilmaz A, Kucuk A, Tumturk A, Menku A. Comparison of dural splitting and duraplasty in patients with Chiari type I malformation: relationship between tonsillo-dural distance and syrinx cavity. Turkish neurosurgery. 2019;29(2):229-236.
21. Chen J, Li Y, Wang T, et al. Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari malformation type I in adult patients: A retrospective analysis of 103 patients. Medicine. 2017;96(4):e5945.
22. Del Gaudio N, Vaz G, Duprez T, Raftopoulos C. Comparison of dural peeling versus duraplasty for surgical treatment of Chiari type I malformation: results and complications in a monocentric patients' cohort. World neurosurgery. 2018;117:e595-e602.
23. Klekamp J. Surgical treatment of Chiari I malformation-analysis of intraoperative findings, complications, and outcome for 371 foramen magnum decompressions. Neurosurgery. 2012;71(2):365-380.
24. Grahovac G, Pundy T, Tomita T. Chiari type I malformation of infants and toddlers. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2018;34(6):1169-1176.
25. da Silva JA, Holanda MM. Basilar impression, Chiari malformation and syringomyelia: a retrospective study of 53 surgically treated patients. Arquivos de neuro-psiquiatria. 2003;61(2b):368-375.
26. Vidal CHF, Brainer-Lima AM, Valença MM, Farias RL. Chiari 1 malformation surgery: comparing non-violation of the arachnoid versus arachnoid opening and thermocoagulation of the tonsils. World neurosurgery. 2018;121:e605-e613.
27. Kunert P, Janowski M, Zakrzewska A, Marchel A. Comparison of results between two different techniques of cranio-cervical decompression in patients with Chiari I malformation. Neurologia i Neurochirurgia Polska. 2009;43(4):337-345.
28. Park YS, Kim DS, Shim KW, Kim JH, Choi JU. Factors contributing improvement of syringomyelia and surgical outcome in type I Chiari malformation. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2008;25(4):453-459.
29. Koueik J, Sandoval-Garcia C, Kestle JRW, et al. Outcomes in children undergoing posterior fossa decompression and duraplasty with and without tonsillar reduction for Chiari malformation type I and syringomyelia: A pilot prospective multicenter cohort study. Journal of Neurosurgery: Pediatrics. 2020;25(1):21-29.
30. Jia C, Li H, Wu J, et al. Comparison decompression by duraplasty or cerebellar tonsillectomy for Chiari malformation-I complicated with syringomyelia. Clinical neurology and neurosurgery. 2018;176:1-7.
31. Wang L, Zhao H, Zhu W, Yan P, Teng YD. A combinatorial approach with cerebellar tonsil suspension to treating symptomatic Chiari malformation type I in adults: a retrospective study. World neurosurgery. 2020;143:e19-e35.
32. Stanko KM, Lee YM, Rios J, et al. Improvement of syrinx resolution after tonsillar cautery in pediatric patients with Chiari type I malformation. Journal of neurosurgery Pediatrics. 2015;17(2):174-181.
33. Asgari S, Engelhorn T, Bschor M, Sandalcioglu IE, Stolke D. Surgical prognosis in hindbrain related syringomyelia. Acta neurologica Scandinavica. 2003;107(1):12-21.
34. Anderson RC, Dowling KC, Feldstein NA, Emerson RG. Chiari I malformation: potential role for intraoperative electrophysiologic monitoring. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2003;20(1):65-72.
35. Barzilai O, Roth J, Korn A, Constantini S. The value of multimodality intraoperative neurophysiological monitoring in treating pediatric Chiari malformation type I. Acta neurochirurgica. 2015;158(2):335-340.
36. Anderson RC, Emerson RG, Dowling KC, Feldstein NA. Improvement in brainstem auditory evoked potentials after suboccipital decompression in patients with Chiari I malformations. Journal of neurosurgery. 2003;98(3):459-464.
37. Zamel K, Galloway G, Kosnik EJ, Raslan M, Adeli A. Intraoperative neurophysiologic monitoring in 80 patients with Chiari I malformation: role of duraplasty. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2009;26(2):70-75.
38. Rasul FT, Matloob SA, Haliasos N, Jankovic I, Boyd S, Thompson DNP. Intraoperative neurophysiological monitoring in paediatric Chiari surgery-help or hindrance? Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2019;35(10):1769-1776.
39. Roser F, Ebner FH, Liebsch M, Tatagiba MS, Naros G. The role of intraoperative neuromonitoring in adults with Chiari I malformation. Clinical neurology and neurosurgery. 2016;150:27-32.
40. Hale AT, Adelson PD, Albert GW, et al. Factors associated with syrinx size in pediatric patients treated for Chiari malformation type I and syringomyelia: a study from the Park-Reeves Syringomyelia Research Consortium. Journal of neurosurgery Pediatrics. 2020:1-11.
41. El-Ghandour NM. Long-term outcome of surgical management of adult Chiari I malformation. Neurosurgical review. 2012;35(4):537-546; discussion 546-537.
42. Soleman J, Roth J, Bartoli A, Rosenthal D, Korn A, Constantini S. Syringo-subarachnoid shunt for the treatment of persistent syringomyelia following decompression for Chiari type I malformation: surgical results. World neurosurgery. 2017;108:836-843.
43. Arnautovic KI, Qaladize BF, Pojskic M, Gienapp AJ, Splavski B, Boop FA. The 270° circumferential microsurgical decompression of the foramen magnum in adult Chiari malformation type I: single surgeon series of 130 patients with syringomyelia, neurologic, and headache outcomes. World neurosurgery. 2020.
44. Ghanem IB, Londono C, Delalande O, Dubousset JF. Chiari I malformation associated with syringomyelia and scoliosis. Spine. 1997;22(12):1313-1317; discussion 1318.
45. Spena G, Bernucci C, Garbossa D, Valfrè W, Versari P. Clinical and radiological outcome of craniocervical osteo-dural decompression for Chiari I-associated syringomyelia. Neurosurgical review. 2010;33(3):297-303; discussion 303-294.
46. Kemerdere R, Akgun MY, Cetintas SC, Kacira T, Tanriverdi T. Clinical and radiological outcomes of arachnoid-preseving suboccipital decompression for adult chiari I malformation with and without syringomyelia. Clinical neurology and neurosurgery. 2019;188:105598.
47. Kennedy BC, Nelp TB, Kelly KM, et al. Delayed resolution of syrinx after posterior fossa decompression without dural opening in children with Chiari malformation type I. Journal of neurosurgery Pediatrics. 2015;16(5):599-606.
48. Nagoshi N, Iwanami A, Toyama Y, Nakamura M. Factors contributing to improvement of syringomyelia after foramen magnum decompression for Chiari type I malformation. Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association. 2014;19(3):418-423.
49. Isu T, Sasaki H, Takamura H, Kobayashi N. Foramen magnum decompression with removal of the outer layer of the dura as treatment for syringomyelia occurring with Chiari I malformation. Neurosurgery. 1993;33(5):845-849; discussion 849-850.
50. Raftopoulos C, Sanchez A, Matos C, Baleriaux D, Bank WO, Brotchi J. Hydrosyringomyelia-Chiari I complex. Prospective evaluation of a modified foramen magnum decompression procedure: preliminary results. Surgical Neurology. 1993;39(2):163-169.
51. Alfieri A, Pinna G. Long-term results after posterior fossa decompression in syringomyelia with adult Chiari type I malformation. Journal of neurosurgery Spine. 2012;17(5):381-387.
52. Attenello FJ, McGirt MJ, Gathinji M, et al. Outcome of Chiari-associated syringomyelia after hindbrain decompression in children: analysis of 49 consecutive cases. Neurosurgery. 2008;62(6):1307-1313; discussion 1313.
53. Takayasu M, Takagi T, Hara M, Anzai M. A simple technique for expansive suboccipital cranioplasty following foramen magnum decompression for the treatment of syringomyelia associated with Chiari I malformation. Neurosurgical review. 2004;27(3):173-177.
54. Bao C, Yang F, Liu L, et al. Surgical treatment of Chiari I malformation complicated with syringomyelia. Experimental and therapeutic medicine. 2012;5(1):333-337.
55. Wu T, Zhu Z, Jiang J, et al. Syrinx resolution after posterior fossa decompression in patients with scoliosis secondary to Chiari malformation type I. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2011;21(6):1143-1150.
56. Xie D, Qiu Y, Sha S, et al. Syrinx resolution is correlated with the upward shifting of cerebellar tonsil following posterior fossa decompression in pediatric patients with Chiari malformation type I. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2014;24(1):155-161.
57. Wetjen NM, Heiss JD, Oldfield EH. Time course of syringomyelia resolution following decompression of Chiari malformation type I. Journal of neurosurgery Pediatrics. 2008;1(2):118-123.
58. Hidalgo ET, Dastagirzada Y, Orillac C, et al. Time to resolution of symptoms after suboccipital decompression with duraplasty in children with Chiari malformation type I. World neurosurgery. 2018;117:e544-e551.
59. Paul KS, Lye RH, Strang FA, Dutton J. Arnold-Chiari malformation. Review of 71 cases. Journal of neurosurgery. 1983;58(2):183-187.
60. Zhang ZQ, Chen YQ, Chen YA, Wu X, Wang YB, Li XG. Chiari I malformation associated with syringomyelia: a retrospective study of 316 surgically treated patients. Spinal cord. 2007;46(5):358-363.
61. Ene CI, Wang AC, Collins KL, et al. Expansile duraplasty and obex exploration compared with bone-only decompression for Chiari malformation type I in children: retrospective review of outcomes and complications. Journal of neurosurgery Pediatrics. 2020:1-8.
62. Krishna V, McLawhorn M, Kosnik-Infinger L, Patel S. High long-term symptomatic recurrence rates after Chiari-1 decompression without dural opening: a single center experience. Clinical neurology and neurosurgery. 2014;118:53-58.
63. Tubbs RS, Beckman J, Naftel RP, et al. Institutional experience with 500 cases of surgically treated pediatric Chiari malformation Type I. Journal of neurosurgery Pediatrics. 2011;7(3):248-256.
64. Balestrino A, Consales A, Pavanello M, et al. Management: opinions from different centers-the Istituto Giannina Gaslini experience. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2019;35(10):1905-1909.
65. De Vlieger J, Dejaegher J, Van Calenbergh F. Multidimensional, patient-reported outcome after posterior fossa decompression in 79 patients with Chiari malformation type I. Surgical neurology international. 2020;10:242.
66. Pomeraniec IJ, Ksendzovsky A, Awad AJ, Fezeu F, Jane JA, Jr. Natural and surgical history of Chiari malformation type I in the pediatric population. Journal of neurosurgery Pediatrics. 2015;17(3):343-352.
67. Klekamp J. Neurological deterioration after foramen magnum decompression for Chiari malformation type I: old or new pathology? Journal of neurosurgery Pediatrics. 2012;10(6):538-547.
68. Kennedy BC, Kelly KM, Phan MQ, et al. Outcomes after suboccipital decompression without dural opening in children with Chiari malformation Type I. Journal of neurosurgery Pediatrics. 2015;16(2):150-158.
69. Flynn JM, Sodha S, Lou JE, et al. Predictors of progression of scoliosis after decompression of an Arnold Chiari I malformation. Spine. 2004;29(3):286-292.
70. Feghali J, Xie Y, Chen Y, Li S, Huang J. The SHORE score: a novel predictive tool for improvement after decompression surgery in adult Chiari malformation type I. World neurosurgery. 2020;142:e195-e202.
71. Attenello FJ, McGirt MJ, Garcés-Ambrossi GL, Chaichana KL, Carson B, Jallo GI. Suboccipital decompression for Chiari I malformation: outcome comparison of duraplasty with expanded polytetrafluoroethylene dural substitute versus pericranial autograft. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2008;25(2):183-190.
72. Gilmer HS, Xi M, Young SH. Surgical decompression for Chiari malformation type I: an age-based outcomes study based on the Chicago Chiari Outcome Scale. World neurosurgery. 2017;107:285-290.
73. Singhal GD, Singhal S, Agrawal G, Singhal D, Arora V. Surgical experience in pediatric patients with Chiari-I malformations aged ≤18 years. journal of neurosciences in rural practice. 2019;10(1):85-88.
74. Pepper J, Elhabal A, Tsermoulas G, Flint G. Symptom outcome after craniovertebral decompression for Chiari type 1 malformation without syringomyelia. Acta neurochirurgica. 2020;163(1):239-244.
75. Guyotat J, Bret P, Jouanneau E, Ricci AC, Lapras C. Syringomyelia associated with type I Chiari malformation. A 21-year retrospective study on 75 cases treated by foramen magnum decompression with a special emphasis on the value of tonsils resection. Acta neurochirurgica. 1998;140(8):745-754.
76. Soleman J, Bartoli A, Korn A, Constantini S, Roth J. Treatment failure of syringomyelia associated with Chiari I malformation following foramen magnum decompression: how should we proceed? Neurosurgical review. 2018;42(3):705-714.
77. Alzate JC, Kothbauer KF, Jallo GI, Epstein FJ. Treatment of Chiari I malformation in patients with and without syringomyelia: a consecutive series of 66 cases. Neurosurgical focus. 2006;11(1):E3.
78. Imperato A, Seneca V, Cioffi V, Colella G, Gangemi M. Treatment of Chiari malformation: who, when and how. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2011;32 Suppl 3:S335-339.
Appendix I. Literature searches
See Chapter 1, Appendix I.
Appendix II. Rating evidence quality
Classification of Evidence on Therapeutic Effectiveness and Levels of Recommendation
Class I evidence
Level I (or A) recommendation |
Evidence from one or more well-designed, randomized controlled clinical trial, including overviews of such trials. |
Class II evidence
Level II (or B) recommendation |
Evidence from one or more well-designed comparative clinical studies, such as non-randomized cohort studies, case-control studies, and other comparable studies, including less well-designed randomized controlled trials. |
Class III evidence
Level III (or C) recommendation |
Evidence from case series, comparative studies with historical controls, case reports, and expert opinion, as well as significantly flawed randomized controlled trials. |
Classification of Evidence on Prognosis and Levels of Recommendation
Class I evidence
Level I (or A) recommendation |
All 5 technical criteria above are satisfied |
Class II evidence
Level II (or B) recommendation |
Four of 5 technical criteria are satisfied |
Class III evidence
Level III (or C) recommendation |
Everything else |
Classification of Evidence on Diagnosis and Levels of Recommendation
Class I evidence
Level I (or A) recommendation |
Evidence provided by one or more well-designed clinical studies of a diverse population using a “gold standard” reference test in a blinded evaluation appropriate for the diagnostic applications and enabling the assessment of sensitivity, specificity, positive and negative predictive values, and, where applicable, likelihood ratios |
Class II evidence
Level II (or B) recommendation |
Evidence provided by one or more well-designed clinical studies of a restricted population using a “gold standard” reference test in a blinded evaluation appropriate for the diagnostic applications and enabling the assessment of sensitivity, specificity, positive and negative predictive values, and, where applicable, likelihood ratios |
Class III evidence
Level III (or C) recommendation |
Evidence provided by expert opinion or studies that do not meet the criteria for the delineation of sensitivity, specificity, positive and negative predictive values, and, where applicable, likelihood ratios |
Classification of Evidence on Clinical Assessment and Levels of Recommendation
Class I evidence
Level I (or A) recommendation |
Evidence provided by one or more well-designed clinical studies in which interobserver and/or intraobserver reliability is represented by a kappa statistic >0.60 |
Class II evidence
Level II (or B) recommendation |
Evidence provided by one or more well-designed clinical studies in which interobserver and/or intraobserver reliability is represented by a kappa statistic >0.40 |
Class III evidence
Level III (or C) recommendation |
Evidence provided by one or more well-designed clinical studies in which interobserver and/or intraobserver reliability is represented by a kappa statistic <0.40 |
Appendix III. PRISMA flowcharts
Question 3-1
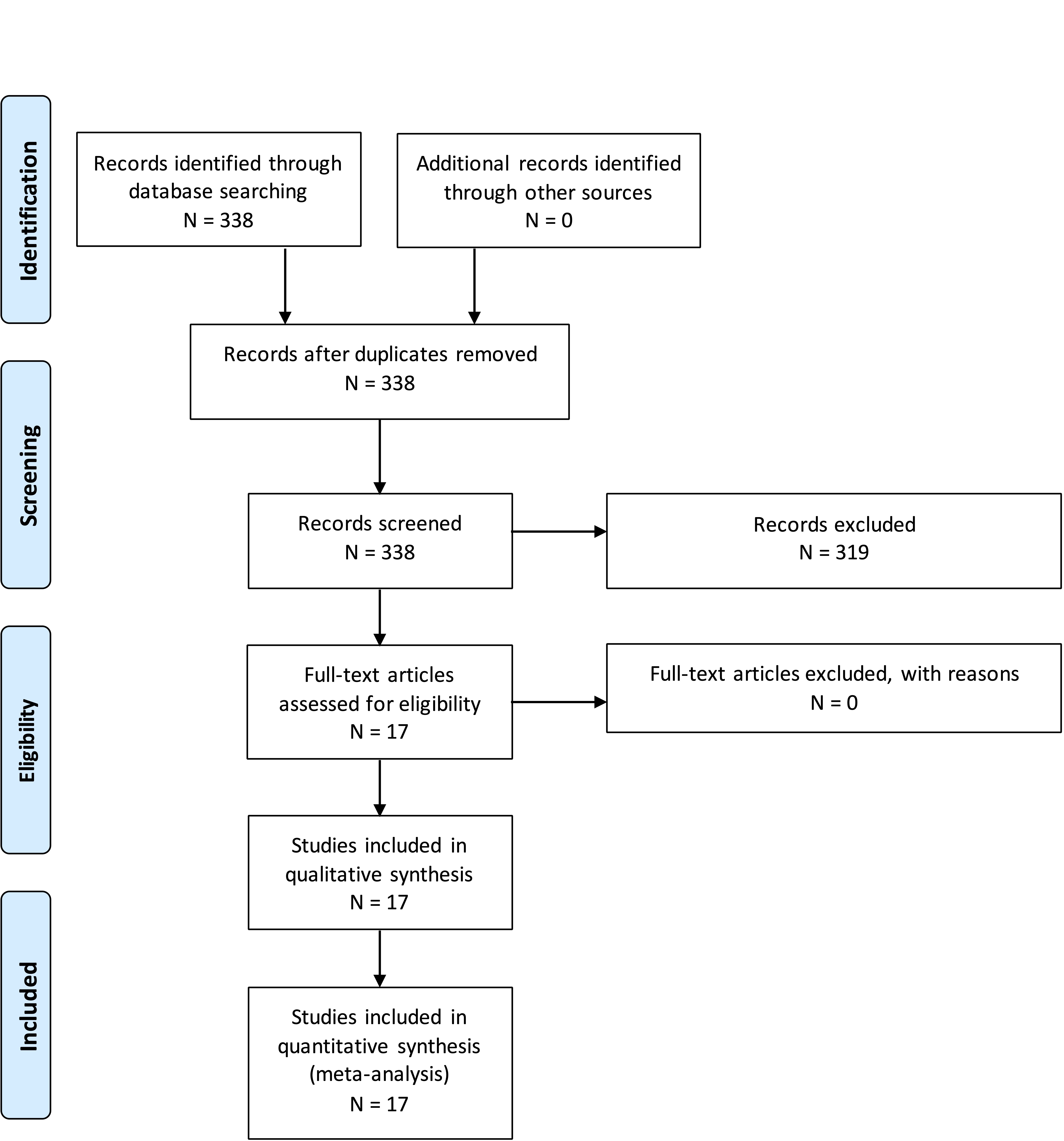
Question 3-2
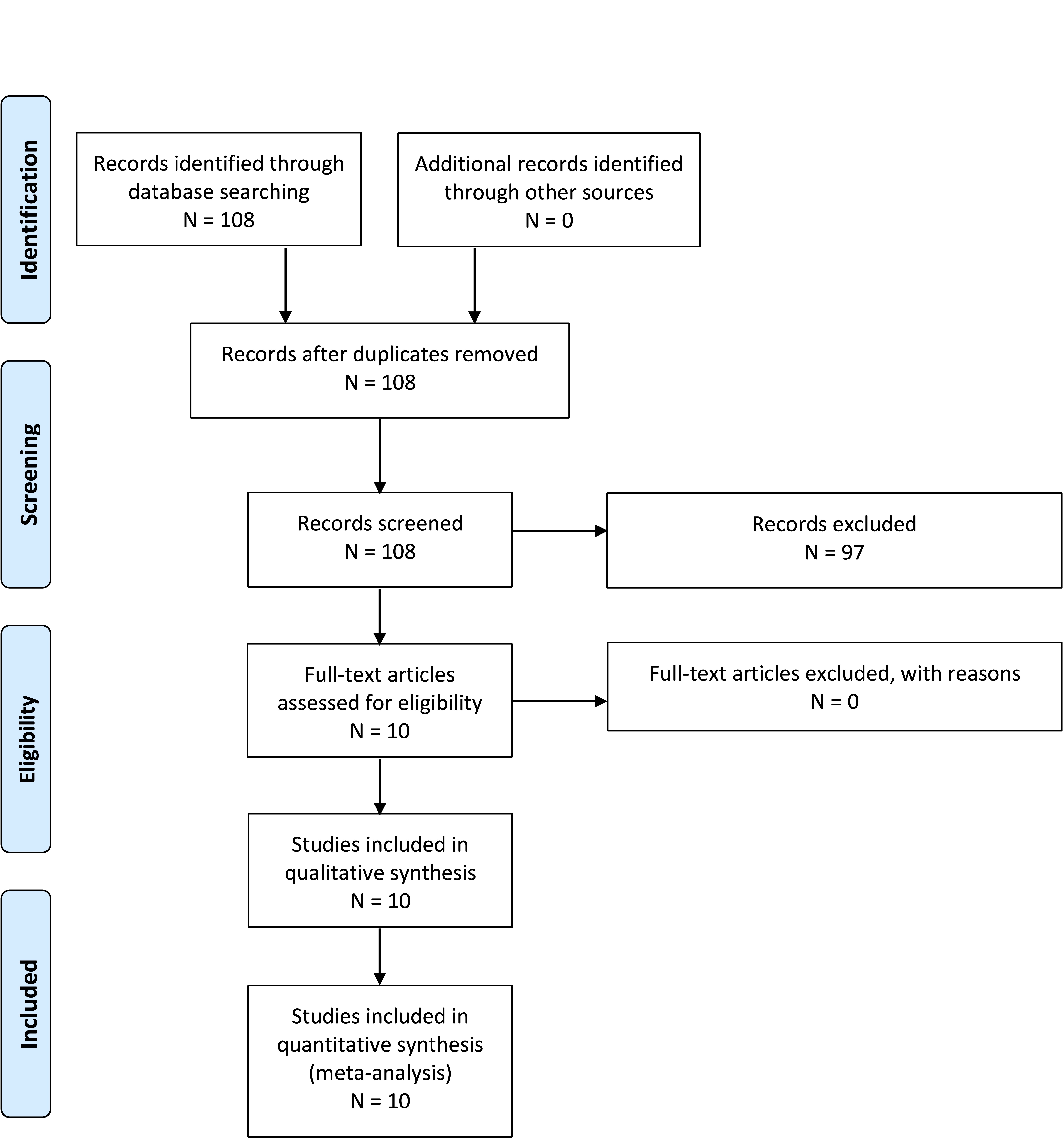
Question 3-3
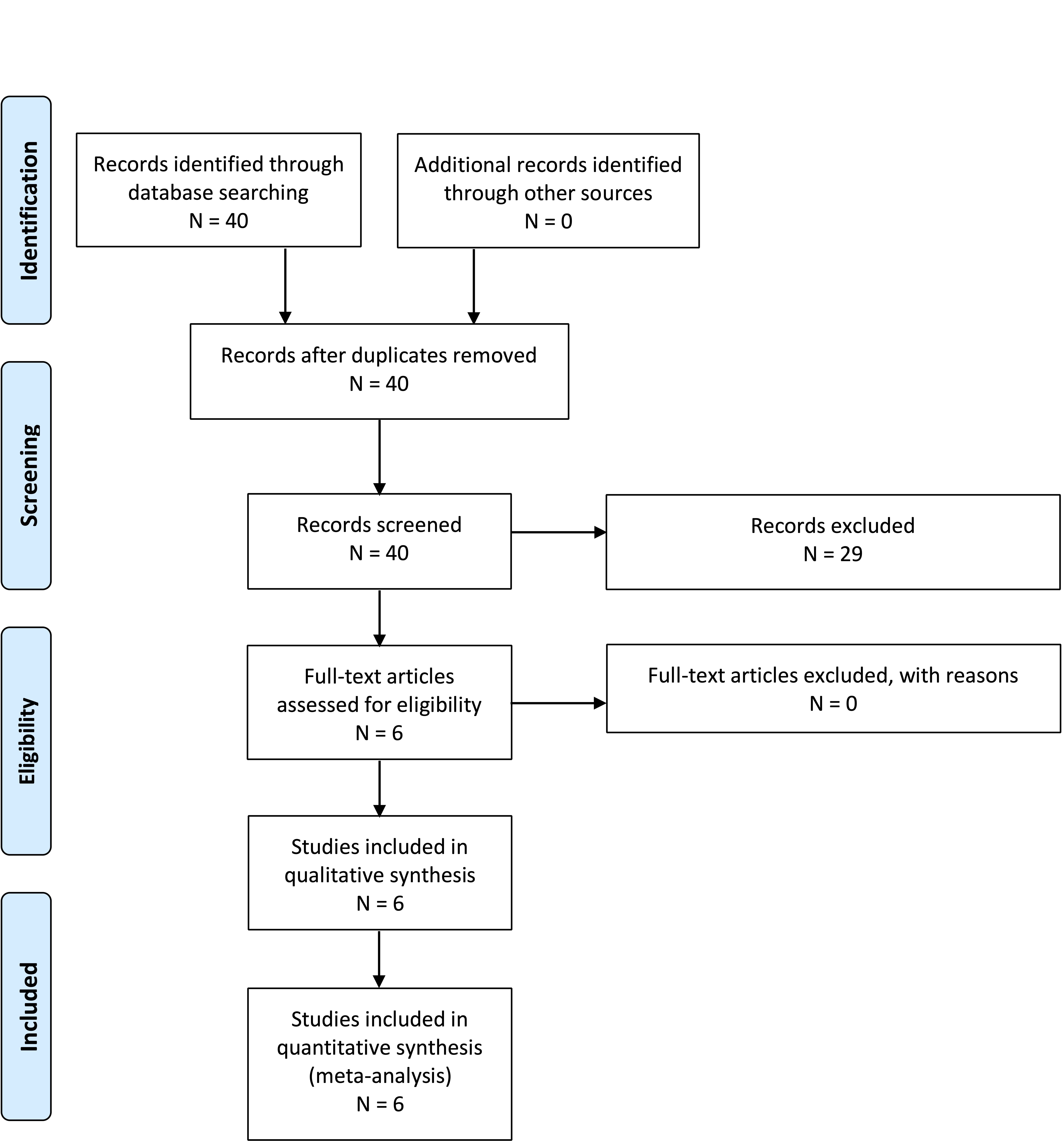
Question 3-4
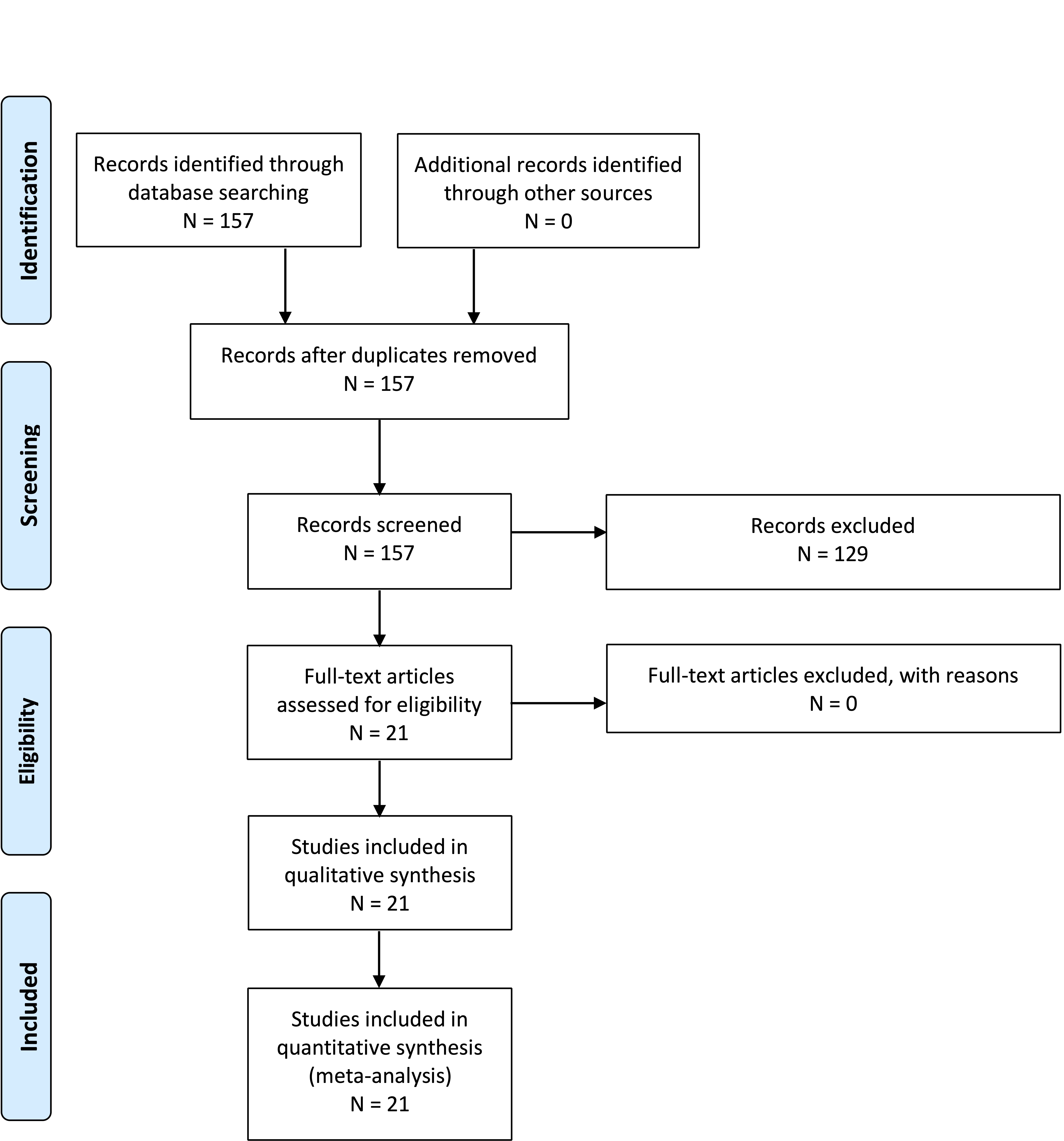
Question 3-5
Appendix IV. Evidence tables
CI, confidence interval; CMD, circumferential microsurgical decompression; CSF, cerebrospinal fluid; CTC, cerebellar tonsil coagulation; CTS, cerebellar tonsil suspension; FMD, foramen magnum decompression; IQR, interquartile range; IOM, intraoperative monitoring; IONM, intraoperative neuromonitoring; MRI, magnetic resonance imaging; PFD, posterior fossa decompression; PFDD, posterior fossa decompression and duraplasty; PFDD-T, posterior fossa decompression and duraplasty with tonsillar resection; RCT, randomized controlled trial; SM, syringomyelia; SSS, syringo-subarachnoid shunt.
| PICO |
Author |
Literature Type |
Description of the study |
Class of Evidence |
Author Conclusions |
| 1 |
Yilmaz et al, 201718 |
Therapy |
Retrospective case series |
III |
Single-center retrospective review that showed there was no difference in outcome between PFD and PFDD, except in patients with severe tonsillar herniation who had an improvement in their cervical motility without compromising their spinal stability |
| 1 |
Ito et al, 202019 |
Therapy |
Retrospective case series |
III |
This study shows increased complications with PFDD, and PFD is not as effective with less syrinx resolution and increased need for reoperation. However, very small subgroups were reported (n = 8 in the duraplasty group) |
| 1 |
Grahovac et al, 201824 |
Therapy |
Retrospective comparative |
III |
In young patients (<3 years of age) with CIM, recurrence rates were higher after a PFD than after a PFDD. The authors reported that PFDD at primary or redo surgery provides for better decompression and long-term outcome. However, this was a small study (n = 16) |
| 1 |
Jiang et al, 201814 |
Therapy |
Prospective comparative RCT |
III |
Found that PFD produces comparable radiologic and clinical outcomes and is associated with a lower risk of complications (higher incidence of CSF leak in the PFDD group) |
| 1 |
Del Gaudio et al, 201822 |
Therapy |
Retrospective case series |
III |
Small numbers (n = 28) but concluded that PFD (with dural peeling) was less risk than PFDD but had a lower response rate (66.7% vs 100%) |
| 1 |
Oral et al, 201920 |
Therapy |
Retrospective case control |
III |
Found that a syrinx cavity is more likely to regress in patients who undergo PFDD, but there are complications with a PFDD. Therefore, they concluded PFD should be initially preformed in patients with CIM and a small syrinx cavity, or those without syringomyelia |
|
| 1 |
Chen et al, 201721 |
Therapy |
Retrospective case control |
III |
No statistically significant differences were found between PFD and PFD groups with regard to demographics, preoperative symptoms, radiographic characteristics, and clinical outcomes. However, postoperative aseptic meningitis occurred more frequently in the PFDD group than the PFD group |
| 1 |
Butensky et al, 202015 |
Therapy |
Retrospective case control |
III |
Found that there is a role for PFDD in patients with severe syringomyelia, but overall PFD may be safely offered as the initial surgical intervention for symptomatic CIM patients |
| 1 |
Gurbuz et al, 20159 |
Therapy |
Retrospective case control |
III |
Reported that in CIM cases with syringomyelia and tonsillar herniation >10 mm and whose symptoms lasted <36 months, PFDD is a more reliable choice than PFD despite a slightly higher rate of complications |
| 1 |
Gallo et al, 201710 |
Therapy |
Retrospective case control |
III |
PFD has comparable clinical and radiologic outcomes to PFDD in children with CIM. But there was a higher risk of postoperative complications with PFDD that was not statistically significant |
| 1 |
Shimoji et al, 201913 |
Therapy |
Retrospective case control |
III |
Small study (n = 26), but PFD was similar to PFDD in terms of the results of surgery and had a lower risk of complications for the treatment of noncomplicated CIM |
| 1 |
Bao et al, 201516 |
Therapy |
Retrospective case series |
III |
Did not compare 2 techniques but did show that PFDD is a rational surgical approach with beneficial clinical effects |
| 1 |
Pisapia et al, 20178 |
Therapy |
Retrospective comparative |
II |
Large study from 1 center. Concluded that equivalent rates of symptom resolution and reoperation after PFDD and PFD support the PFD approach as a first-line surgical option for pediatric CIM patients |
| 1 |
Pandey et al, 202012 |
Therapy |
Retrospective case series |
III |
Showed more optimal syrinx resolution, shorter operative time, and shorter postoperative stay with the PFDD group. Both PFD and PFDD had similar outcome scores |
| 1 |
Klekamp et al, 201223 |
Therapy |
Retrospective case series |
III |
Series of 371 patients only included PFDD, but it offered a favorable long-term prognosis. However, there was a high complication rate of 21.8%, permanent morbidity rate of 3/2%, and mortality rate of 1.3% |
| 1 |
Chotai et al, 202017 |
Therapy |
Retrospective case series |
III |
Maximal reduction in syrinx size can be expected within 3 months after PFD in patients with SM and a syrinx; however, the syringes continue to regress over time. In patient with CIM and SM, the median time to >50% regression in maximal syrinx diameter was 8 months |
| 1 |
Yilmaz et al, 201111 |
Therapy |
Retrospective comparative |
III |
In patients with tonsillar descent below C1, PFDD may lead to a more reliable decrease in SM and outcome scores, but with less severe tonsillar descent, PFD alone may be performed |
| 1 |
da Silva et al, 200325 |
Therapy |
Case series |
III |
Included 53 patients 24 without tonsil manipulation. Symptom improvement was shown |
| 2 |
Vidal et al, 201926 |
Therapy |
RCT |
III |
Included 32 patients, with 16 in each group. There were more complications in the tonsil coagulation group |
| 2 |
Wang et al, 202031 |
Therapy |
Comparative |
III |
Included 42 patients, 12 of whom had PFDD, 13 had PFDD with coagulation and 17 had PFDD with coagulation and suspension. Patients who underwent PFDD + CTC + CTS improved more than comparison group |
| 2 |
Kunert et al, 200927 |
Therapy |
Comparative |
III |
Included 38 patients with 6 months postoperative MRI. Results of PFD and PFDD are comparable, but the risk of post op complications after PFD were significantly lower |
| 2 |
Park et al, 200928 |
Therapy |
Case series |
III |
Included 57 patients 40 of whom had PFD. Seventeen patients had PFD with tonsil management. There was no difference in outcomes |
| 2 |
Stanko et al, 201632 |
Therapy |
Case series |
III |
Included 171 patients, 43 with tonsil cautery and 128 without. Syrinx resolution more likely with cautery, no increase in complications |
| 2 |
Koueik et al, 202029 |
Therapy |
Comparative |
III |
Included 75 patients, and 42 had PFDD-T, while 26 had PFDD. This was a prospective, nonrandomized study and there was no difference in outcomes or complications |
| 2 |
Chotai et al, 202017 |
Therapy |
Case series |
III |
Included 85 patients, 5 of whom had bone one only, 21 had tonsil cauterization and 15 had arachnoid veil transection. Resolution of syringomyelia was more likely when tonsil manipulation was performed |
| 2 |
Jia et al, 201830 |
Therapy |
Retrospective comparative |
III |
In 115 adult patients, there were 37 with posterior fossa decompression with duraplasty and 78 who had duraplasty and cerebellar tonsil resection. There was no difference in symptom resolution or syrinx reduction between groups. There was higher complication rate for subjects who had tonsil resection |
| 2 |
Asgari et al, 200333 |
Therapy |
Retrospective comparative |
III |
Nonrandomized. Technique difference was due to different preferences by different surgeons. No overlap |
| 3 |
Anderson et al, 200334 |
Therapy |
Retrospective case series |
III |
There was no comparison |
| 3 |
Anderson et al, 200336 |
Therapy |
Prospective case series |
III |
No comparison. Authors concluded that in pediatric patients the majority of improvement occurs after bone decompression and division of the atlanto-occipital membrane, rather than opening of the dura |
| 3 |
Zamel et al 200937 |
Therapy |
Retrospective case series |
III |
No comparison. Data revealed that for both groups of patients, with or without associated syringomyelia, the predominant improvement in central conduction in most cases occurred during the period of bony decompression without significant additional improvement after the duraplasty procedure |
| 3 |
Rasul et al, 201938 |
Therapy |
Retrospective case series |
III |
No comparison. Found no link between clinical outcomes and IONM, nor did syrinx outcome correlate with IONM |
| 3 |
Roser et al, 201639 |
Patient assessment |
Retrospective case series |
III |
A 39-patient case series with no comparison group. All patients had IOM with CMD. Authors fail to show much if any effectiveness |
| 3 |
Barzilai et al, 201635 |
Diagnostic test |
Retrospective case series |
III |
There was no comparison group |
| 4 |
Arnautovic et al, 202043 |
Patient assessment |
Prospective case series |
II |
Reports all syrinx cases stable or reduced in size by 8 months. No patients in the syrinx subgroup required additional surgery. Of 43 patients with syrinx, 35 resolved and 8 improved. MRI was performed at 2, 6, and 12 months. The median time to resolving/improving syrinx was 4 months (IQR, 3-8 months) |
| 4 |
Ghanem et al, 199744 |
Patient assessment |
Case series |
III |
In 11 of 12 patients, syrinxes improved by 1 year. The 1 case without radiographic improvement had clinical improvement. There were no reoperations for failure to improve syrinx |
| 4 |
Spena et al, 201045 |
Patient assessment |
Retrospective case series |
III |
Postoperative MRI was performed at 3 and 6 months and then annually. All patients had at least 3 postoperative MRI scans. Syringomyelia improved in 29 patients (80.5%) and remained unchanged in 7 (19.4%). The mean time for syrinx reduction was 8 months (range, 6-26 months). |
| 4 |
Kemerdere et al, 202046 |
? |
Retrospective case series |
III |
Follow-up MRI performed at 3 and 12 months. There was a decrease in size in all syrinxes 3 months after surgery. Long-term follow-up showed persistent improvement in 16 of 21 patients (76.1%) |
| 4 |
Kennedy et al, 201547 |
? |
Retrospective case series |
III |
Fifty-seven patients with available images had mean radiographic follow-up of 32 months. Of these, 70% (40) demonstrated radiographic improvement on MRI |
| 4 |
Hale et al, 202040 |
? |
Retrospective case series |
I |
Kaplan-Meier analysis of time to syrinx resolution (<2 mm diameter) shows 50% resolution by slightly over 3 years. By 6 years, nearly all syringomyelia had improved |
| 4 |
Nagoshi et al, 201448 |
? |
Retrospective case series |
III |
Authors recommend a minimum of 12 months’ follow-up before considering additional intervention |
| 4 |
Isu et al, 199349 |
? |
Retrospective case series |
III |
In a series of 7 patients, 5 syrinx decreased over weeks and 2 decreased over months |
| 4 |
Raftopoulos et al, 199350 |
? |
Retrospective case series |
III |
MRI follow-up was performed 9 days, 2 months, 6 months after surgery, and then annually. By 2 months, all 8 patients had decreased syrinx volume. All were then stable or further decreased on subsequent imaging |
| 4 |
Stanko et al, 201632 |
? |
Retrospective case series |
III |
MRI images were obtained 3–6 months after surgery. If there was no improvement, imaging was repeated 3–4 months after the first postoperative scan. The mean time to syrinx improvement was 11 months. |
| 4 |
El-Ghandour et al, 201241 |
? |
Retrospective case series |
III |
Postoperative MRI performed every 3 months until syrinx resolution or until 18 months. The mean time to syrinx resolution was 7.4 months |
| 4 |
Alfieri et al, 201251 |
? |
Retrospective case series |
II |
MRI performed at 6 weeks and 6 months after surgery, and then annually. At 1 year, 29% had syrinx resolution; 57% had unchanged syrinx size. Results were similar at last follow-up |
| 4 |
Attenello et al, 200852 |
? |
Retrospective case series |
II |
Three-month postoperative MRI, usually repeated 12 to 18 months after surgery. The median time to radiographic improvement was 14 months after surgery |
| 4 |
Takayasu et al, 200453 |
? |
Retrospective case series |
III |
Postoperative MRI was performed between 1 week and 3 months after surgery. The second postoperative MRI was performed at 3, 4, and 7 months. Syrinx reduced in size in all patients by 7 months |
| 4 |
Bao et al, 201354 |
? |
Retrospective case series |
III |
One hundred forty-eight patients received MRI within 2 weeks after operation. Seventy-five patients (50.7%) had a reduced spinal syrinx |
| 4 |
Wu et al, 201255 |
? |
Retrospective case series |
III |
Follow-up MRI was conducted at 6 months and 2, 4, and 6 years postsurgery. Thirty-six of 44 patients (81.8%) had significant improvement within 6 months after surgery, and 97.7% (43/44) had significant improvement by the final follow-up |
| 4 |
Xie et al, 201556 |
? |
Retrospective case series |
III |
Of 87 patients, 31 cases who had both 6-month and a >12-month follow-up were selected to evaluate the time course of syrinx resolution. Most syrinx improvement noted by 6 months then slow improvement over the next several years |
| 4 |
Wetjen et al, 200857 |
? |
Retrospective case series |
II |
The median time to syrinx narrowing (>50% reduction in syrinx diameter) was 3.6 months (95% CI, 3-6.5 months) |
| 4 |
Hidalgo et al, 201858 |
? |
Retrospective case series |
III |
MRI was obtained at 3-, 6-, 12-, 18-, and 24-month intervals. The median time to syrinx improvement was 3 months (range, 3-72 months) |
| 4 |
Chotai et al, 202017 |
? |
Retrospective |
II |
Maximum syrinx regression was seen at 3 months but noted to improve for as long as 12 years |
| 4 |
Soleman et al, 201742 |
? |
Retrospective case series |
III |
The average time from FMD to SSS for patients in this study was 3 year ± 3 years (range, 134-4104 days) |
| 5 |
Paul et al, 198359 |
Therapy |
Retrospective case series |
III |
Seventy-one patient series showed 21% symptom recurrence/deterioration after initial improvement 2-3 years out from surgery, no reoperation data |
| 5 |
Zhang et al, 200860 |
Therapy |
Retrospective case series |
III |
Three hundred sixteen patient series with 7% syrinx enlargement at ≥2 years of follow-up |
| 5 |
Ene et al, 202061 |
Therapy |
Retrospective case series |
III |
Two hundred seventy-six patients followed for symptom and syrinx resolution and scoliosis outcomes mean follow-up 35 months without reoperation data |
| 5 |
Pisapia et al, 20178 |
Therapy |
Retrospective case series |
III/II |
One hundred eighty-nine patients followed up 1-75 months showed 8% reoperation. Unclear what findings for reoperation were found in short- vs. long-term follow-up |
| 5 |
Krishna et al, 201462 |
Therapy |
Retrospective case series |
III |
Forty-seven patients showed 31.9% of patients had redo decompression (<1 year to 11 years), 22.9% reoperation for Chiari associated; 31.9% reoperation for recurrent symptoms at mean 2.6 years 1-11 postoperative from initial surgery FMD |
| 5 |
Tubbs et al, 201163 |
Therapy |
Retrospective case series |
III |
Five hundred cases followed 2 months to 15 years, mean 5 years, 15 patients (3%) reoperated for syrinx or symptoms |
| 5 |
Alfieri et al, 201251 |
Therapy |
Retrospective case series |
III |
One hundred nine patients median follow-up of 12.7 years, followed-up at 1 year and last follow-up for spinal sx, pain, cranial sx, and syrinx. Beyond 1 year at last follow-up, 2 syrinx expansions and 1 worsening pain without reoperation |
| 5 |
Balestrino et al, 201964 |
Therapy |
Retrospective case series |
III |
One hundred seventy-two patients mean follow-up 5.1 years. Six patients had (3.5%) redo decompression without timeline details short vs. long |
| 5 |
De Vlieger et al, 201965 |
Therapy |
Retrospective case series |
III |
Seventy-nine patients were interviewed, with median follow-up 5.8 years. 2 (2.5%) patients with syrinx recurrence at 2 years were reoperated |
| 5 |
Pomeraniec et al, 201666 |
Therapy |
Retrospective case series |
III |
Mean follow-up 66 months, 25 surgical patients. There was no reoperation data |
| 5 |
Klekamp et al, 201267 |
Therapy |
Retrospective case series |
III |
Of 371 patients, 45 (12%) had reoperations. Patients were followed long term 5 and 10 years with patient deterioration |
| 5 |
Attenello et al, 200852 |
Therapy |
Retrospective case series |
III |
In 49 patients, all but 1 patient improved by 12 months. 1 patient did not go on to have additional surgery |
| 5 |
Kennedy et al, 201568 |
Therapy |
Retrospective case series |
III |
Study included 156 patients with 32 months’ follow-up. Of 14 cases, 9% not improved by 22 months were reoperated on at 3-57 months |
| 5 |
Flynn et al, 200469 |
Therapy |
Retrospective case series |
III |
Patients followed long-term for scoliosis progression after Chiari decompression. Patients were followed 2-15 years |
| 5 |
Feghali et al, 202070 |
Therapy |
Retrospective case series |
III |
Of 149 patients, mean follow-up was 1.9 years. There was no reoperation for syrinx progression |
| 5 |
Attenello et al, 200971 |
Therapy |
Retrospective case series |
III |
Of 67 patients, 4 (6%) patients required revision on average 16 months postoperatively |
| 5 |
Gilmer et al, 201772 |
Therapy |
Retrospective case series |
III |
One hundred forty-four patients had a mean follow-up 27 months following symptoms. There was no reoperation data |
| 5 |
Singhal et al, 201973 |
Therapy |
Retrospective case series |
III |
Of 50 patients, 8 (1.6%) patients were reoperated on at 24 months. Two patients referred for scoliosis surgery |
| 5 |
Pepper et al, 202174 |
Therapy |
Retrospective case series |
III |
One hundred twenty-nine patients had at least 24 months average follow-up. There were no revision data |
| 5 |
Guyotat et al, 199875 |
Therapy |
Retrospective case series |
III |
Of 75 patients, 27 (36%) reoperated patients in the follow-up timeframe |
| 5 |
Wetjen et al, 200857 |
Therapy |
Retrospective case series |
III |
In 29 patients, 96% showed symptoms improved by 6 months, no deterioration >1 year |
| 5 |
Hidalgo et al, 201858 |
Therapy |
Retrospective case series |
III |
In 105 patients, there were 12 (1.1%) reoperations at mean 8.9 months postoperatively (range, 1.3-77 months). Median time to syrinx improvement was 3 months (range, 3-72 months) |
| 5 |
Chotai et al, 202017 |
Therapy |
Retrospective case series |
III |
In 85 patients, there was reoperation in 3 (3.5%) patients |
| 5 |
Soleman et al, 201976 |
Therapy |
Retrospective case series |
III |
Of 48 patients, 24 (50%) patients had additional surgery at mean 21.4 months (range, 12 days to 34.9 months) |
| 5 |
Alzate et al, 200177 |
Therapy |
Retrospective case series |
III |
Sixty-six patients had a mean follow- up of 24 months with no mention of reoperations |
| 5 |
Imperato et al, 201178 |
Therapy |
Retrospective case series |
III |
Thirty-six patients were followed up to 18 months from surgery with no reoperations |
Appendix V. Conflicts of Interest
| Name |
Affiliation |
Type of COI |
| Toba Niazi, MD |
Live Like Bella Foundation, Nicklaus Children's Hospital |
Grants/Research Support |
| Laurie Ackerman, MD |
Park Reeves Consortium |
Grants/Research Support |
| David Bauer, MD |
None |
|
| Brandon G. Rocque, MD, MS, FAANS |
None |
|
| Carolyn S. Quinsey, MD |
None |
|
| Eric Jackson, MD |
None |
|
| Jogi V. Pattisapu MD FAAP FAANS |
J&J, Integra |
Consultant |
| Rabia Qaiser, MD |
None |
|
| Cormac O. Maher, MD, FAAP, FACS, FAANS |
None |
|
| Shobhan H. Vachhrajani MD, PhD, FRCSC |
None |
|
| Libby Infinger, MD |
None |
|
| Howard Silberstein, MD |
None |
|
| Sarah Jernigan, MD |
None |
|
| Jeffrey S. Raskin MS MD FAANS FAAP |
None |
|
| Dorothy Poppe |
None |
|
| Kaitlyn Esposito, MPH |
None |
|