Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines for Chiari Malformation: Symptoms
Download PDF Neurosurgery, 2023
Sponsored by: Congress of Neurological Surgeons (CNS) and the Section on Pediatrics
Endorsed by: The Congress of Neurological Surgeons (CNS), American Association of Neurological Surgeons (AANS) and the Bobby Jones Chiari and Syringomyelia Foundation (Bobby Jones CSF)
Authors:
Eric M. Jackson, MD1, Sarah Jernigan, MD, MPH2, Jeffrey S. Raskin MS MD3, Laurie L Ackerman, MD4, Libby Kosnik Infinger, MD, MPH5, Cormac O. Maher, MD, FAAP, FACS, FAANS6, Toba Niazi, MD7, Jogi V. Pattisapu MD FAAP FACS FAANS8, Rabia Qaiser, MD9, Carolyn Quinsey, MD10, Brandon G. Rocque, MD, MS11, Howard Silberstein, MD12, Shobhan Vachhrajani MD, PhD, FRCSC13, David F. Bauer, MD, MPH14
Departmental and institutional affiliations:
- Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD
- Carolina Neurosurgery & Spine Associates, Charlotte, NC
- Department of Neurological Surgery, Northwestern University Feinberg School of Medicine, Chicago, IL
- Department of Neurological Surgery, Indiana University Health, Indianapolis, IN
- Department of Neurosurgery, Medical University of South Carolina (MUSC), Charleston, SC
- Department of Neurosurgery, Stanford Medicine, Palo Alto, CA
- Department of Neurological Surgery, Nicklaus Children's Hospital, Miami, FL
- Pediatric Neurosurgery, University of Central Florida College of Medicine, Orlando FL
- Department of Neurological Surgery, Indiana University School of Medicine, Indianapolis, IN
- Department of Neurosurgery, University of North Carolina Chapel Hill, Chapel Hill, NC
- Division of Pediatric Neurosurgery, Department of Neurosurgery, University of Alabama at Birmingham, Birmingham, AL
- Department of Neurosurgery, University of Rochester School of Medicine and Dentistry, Rochester, NY
- Department of Pediatrics, Wright State University Boonshoft School of Medicine, Dayton, OH
- Department of Neurosurgery, Baylor College of Medicine, Division of Pediatric Neurosurgery, Texas Children’s Hospital, Houston, TX
Corresponding Author contact information:
Eric M. Jackson, MD
Department of Neurosurgery, Johns Hopkins University School of Medicine
Baltimore, MD
ejackson@jhmi.edu
No part of this article has been published or submitted for publication elsewhere.
Keywords:
Abbreviations: CIM, Chiari type I malformation; CSF, cerebrospinal fluid; PFD, polysomnography (PSG); sleep disordered breathing (SDB)
ABSTRACT
Background: Chiari I malformation (CIM) is characterized by descent of the cerebellar tonsils through the foramen magnum, potentially causing symptoms from compression or obstruction of the flow of cerebrospinal fluid (CSF). Diagnosis and treatment of CIM is varied, and guidelines produced through systematic review may be helpful for clinicians.
Objective: We performed a systematic review of the medical literature to answer specific questions on the diagnosis and treatment of CIM.
Methods: PubMed and Embase were queried between 1946 and January 23, 2021 using the search strategies provided in Appendix I.
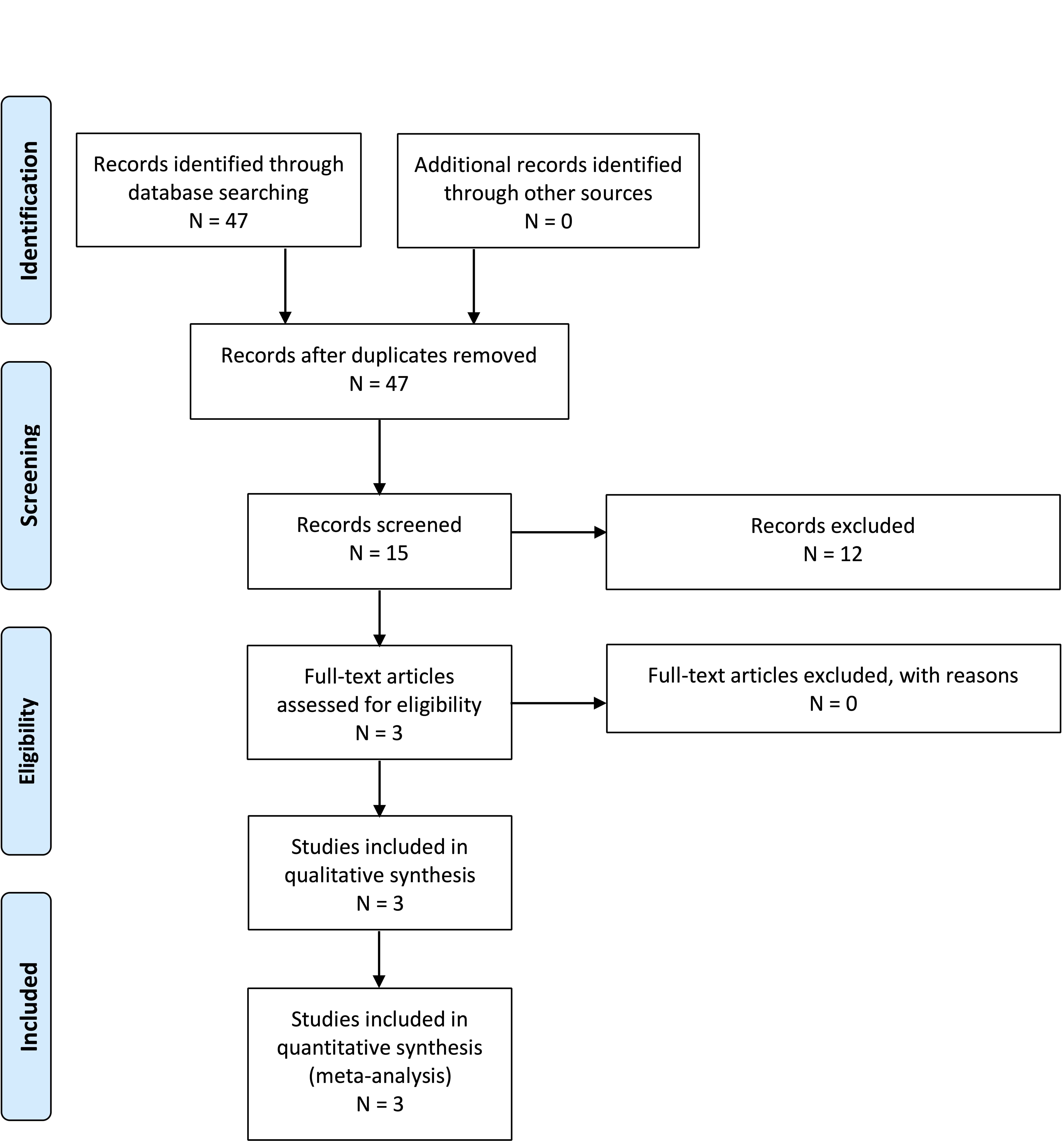
Results: The literature search yielded 430 abstracts, of which 79 were selected for full-text review, 44 were then rejected for not meeting the inclusion criteria or for being off-topic, and 35 were included in this systematic review.
Conclusion: Four Grade C recommendations were made based on Class III evidence and 1 question had insufficient evidence.
RECOMMENDATIONS
2-1. In patients operated for symptomatic CIM, what symptoms are most likely to improve after surgery?
Recommendation: Clinicians may perform foramen magnum decompression surgery on symptomatic patients with CIM to improve pain associated with strain-related headaches. Other symptoms demonstrate more variable response to decompression.
Strength of recommendation: Grade C
Level III evidence
2-2. In patients with asymptomatic CIM without syrinx, is prophylactic surgery indicated to prevent future need for surgery? What is the chance of developing symptoms in the future?
Recommendation: Clinicians should not perform prophylactic surgery on patients with asymptomatic CIM without syrinx. There is a small percentage of patients who develop new or worsening symptoms in the future.
Strength of recommendation: Grade C
Level III evidence
2-3. In patients with asymptomatic CIM without syrinx, should the patient have any activity restrictions to prevent future harm?
Recommendation: Clinicians should not recommend activity restrictions for patients with asymptomatic CIM without syrinx, as there is no evidence of future harm prevention.
Strength of recommendation: Grade C
Level III evidence
2-4. In patients with CIM, should sleep or swallow studies be routinely performed to evaluate for sleep apnea or dysphagia?
Recommendation: There is insufficient evidence to support routine sleep and swallow studies in patients with CIM without sleep or swallow symptoms.
Strength of recommendation: Grade insufficient
2-5. In patients with CIM, should siblings or first-degree relatives be screened for CIM?
Recommendation: Clinicians should not routinely screen asymptomatic siblings or first-degree relatives of patients with CIM.
Strength of recommendation: Grade C
Level III evidence
INTRODUCTION
Goals and Rationale
This clinical guideline has been created to improve patient care by outlining the appropriate information gathering and decision-making processes involved in the treatment of patients with Chiari I malformation (CIM). Care for patients with CIM is provided in many different settings by many different providers. This guideline has been created as an educational tool to guide qualified physicians through a series of diagnostic and treatment decisions to improve the quality and efficiency of care.
Objectives
CIM is a structural abnormality related to the anatomy of the base of the skull and the cerebellum. CIM is defined as descent of the cerebellar tonsils ≥3 to 5 mm below the foramen magnum. Based on a definition of a tonsillar position of ≥5 mm below the foramen magnum, imaging studies estimate a prevalence ranging from 0.24% to 2.6% of the population,1–5 including children and adults. Patients may have varied symptoms and responses to treatment for their CIM, with controversy about what symptoms may relate to the underlying malformation. This chapter aims to provide guidelines based on the literature regarding the symptoms most likely to relate to patients with CIM and thus respond to treatment for CIM, as well as the need for prophylactic surgery, activity restrictions, symptom evaluation, and familial screening.
METHODOLOGY
The guidelines task force initiated a systematic review of the literature and evidence-based guideline relevant to the treatment of patients with CIM. Through objective evaluation of the evidence and transparency in the process of making recommendations, this evidence-based clinical practice guideline was developed for the diagnosis and treatment of patients with CIM. These guidelines are developed for educational purposes to assist practitioners in their clinical decision-making processes. Additional information about the methods used in this systematic review is provided below.
Literature Search
Task force members identified search terms/parameter and a medical librarian implemented the literature search, consistent with the literature search protocol (see Appendix I), using the National Library of Medicine/PubMed database and Embase for the period from 1946 to January 23, 2021 using the search strategies provided in Appendix I.
Inclusion/Exclusion Criteria
Articles were retrieved and included only if they met specific inclusion/exclusion criteria. To reduce bias, these criteria were specified before conducting the literature searches.
Articles that do not meet the following criteria, for the purposes of this evidence-based clinical practice guideline, were excluded. To be included as evidence in the guideline, an article had to be a report of a study that:
- Investigated patients with CIM;
- Studies that enrolled ≥80% of CIM (we included studies with mixed patient populations if they reported results separately for each group/patient population);
- Was a full article report of a clinical study;
- Was not a medical records review, meeting abstract, historical article, editorial, letter, or commentary;
- Appeared in a peer-reviewed publication or a registry report;
- Enrolled a minimum of 10 patients;
- Was of humans;
- Was published in or after 1946;
- Quantitatively presented results;
- Was not an in vitro study;
- Was not a biomechanical study;
- Was not performed on cadavers;
- Was published in English;
- Was not a systematic review, meta-analysis, or guideline developed by others
Systematic reviews or meta-analyses conducted by others, or guidelines developed by others were not included as evidence to support this review because of the differences in article inclusion/exclusion criteria specified compared with the criteria specified by the Guidelines Task Force. Although these articles were not included as evidence to support the review, these articles were recalled for full-text review for the Guidelines Task Force to conduct manual searches of the bibliographies.
Assessment for Risk of Bias
The methodological quality of randomized controlled trials and the risk of bias were assessed using the following 6 criteria:
- Sequence generation (Was the allocation sequence adequately generated?)
- Allocation concealment (Was allocation adequately concealed such that it could not be foretold?)
- Blinding (Were participants, treatment providers and/or outcome assessors blinded to the treatment allocations?)
- Incomplete reporting of data (Were incomplete outcome data adequately addressed?)
- Selective reporting of outcomes (Were all the outcomes specified reported?)
- Other potential threats to validity (Was the randomized controlled trial free of other issues that could put it at a high risk of bias?)
1The guideline task force did not include systematic reviews, guidelines, or meta-analyses conducted by others. These documents are developed using different inclusion criteria than those specified in this guideline; therefore, they may include studies that do not meet the inclusion criteria specific to this guideline. In cases where these types of documents’ abstract suggested relevance to the guideline’s recommendations, the task force searched their bibliographies for additional studies.
In the case of nonrandomized observational evidence, potential threats to the validity of the data were assessed by examining for:
- Bias due to selective case choice for study and selective result reporting
- Bias due to lack or loss of information over time
- The biases of the interpreting investigator regarding the study
- Publication bias regarding positive studies or positive cases
- Misclassification
- Survivorship bias
- Publication bias
- Recognition that in data collected in a retrospective or prospective manner correlation does not imply causation
- Election bias
- Attrition bias
- Bias of change in methods over time
- Ascertainment bias
Rating Quality of Evidence
The quality of evidence was rated using an evidence hierarchy for each of 4 different study types; therapeutic, prognostic, diagnostic, and decision modeling. These hierarchies are shown in Appendix II: Rating Evidence Quality. Additional information regarding the hierarchy classification of evidence can be located here: https://www.cns.org/guidelines/guideline-procedures-policies/guideline-development-methodology.
Revision Plans
In accordance with the Institute of Medicine’s standards for developing clinical practice guidelines and criteria specified by the National Guideline Clearinghouse, the task force will monitor related publications after the release of this document and will revise the entire document and/or specific sections “if new evidence shows that a recommended intervention causes previously unknown substantial harm; that a new intervention is significantly superior to a previously recommended intervention from an efficacy or harms perspective; or that a recommendation can be applied to new populations.”6 In addition, the task force will confirm within 5 years from the date of publication that the content reflects current clinical practice for treatment of CIM.
RESULTS
The literature search yielded 430 abstracts. Task force members reviewed all abstracts yielded from the literature search and identified the literature for full-text review and extraction, addressing the clinical questions, in accordance with the literature search protocol (Appendix I). Task force members identified the best research evidence available to answer the targeted clinical questions. When class I, II and or III literature was available to answer specific questions, the task force did not review class IV studies.
The task force selected 79 full-text articles for full-text review. Of these, 44 were rejected for not meeting the inclusion criteria or for being off-topic. Thirty-five were selected for this systematic review (Appendix III).
DISCUSSION
Question 2-1. In patients operated for symptomatic CIM, what symptoms most likely improve after surgery?
Recommendation: Clinicians may perform foramen magnum decompression surgery on symptomatic patients with CIM to improve pain associated with strain-related headaches. Other symptoms demonstrate more variable response to decompression.
Strength of recommendation: Grade C
Class III Evidence
There is an extensive literature on symptoms related to CIM and likelihood of response to intervention. Studies were all class III evidence and had varying objectives, but symptom response data were abstracted on review. Manuscripts focused on patient outcomes, outcome scores, or clinical decision rules were excluded from analysis based on a lack of focus on specific symptoms for answering this question. Notably, manuscripts often included patients with syringomyelia, but syrinx as a separate entity was not considered to be a symptom for this question. Syringomyelia may affect symptoms attributed to Chiari with 1 study reporting7 variable improvement in symptoms in patients with and without syrinx. For this question, symptoms that may be related to either the CIM or syrinx, including numbness or weakness, are included in the general discussion, as they are difficult to separate. Syrinx is discussed as a separate entity in Chapter 1 of these guidelines.
After review of the literature, no symptoms demonstrated uniform improvement. Headache was the symptom most likely to respond to intervention; strain-induced occipital headache was the most likely to respond. Other headaches, including frontal headaches and non–strain-induced headaches were also noted to show improvement in some cases but to a lesser degree than occipital strain-induced headaches, which have been classically termed “Chiari headaches.”8 Other symptoms including drop attacks; visual symptoms or changes; vestibular dysfunction including hearing loss, tinnitus, or vertigo; extremity numbness, weakness, or dysesthetic pain; and myelopathy and ataxia likewise showed a variable response across the literature but with less consistent improvement, either due to smaller sample size or a much lower percentage of improvement reported.7–27
Age-related differences in symptomatology were identified, with children noted to be more likely to have oropharyngeal symptoms including sleep apnea and reflux.28–30 These symptoms did appear to improve with intervention in patients across different studies.
Although not always discussed as a symptom, 1 study in a limited patient set (11 patients) demonstrated improvement in some patients in neuropsychological testing in areas including executive function, verbal learning, psychomotor speed, and color naming speed.31
The literature on symptoms in CIM is extensive. As all the studies reviewed were class III data, it is difficult to draw significant conclusions. The variability of response of different symptoms in different studies is subject to many confounding factors. As such, there is not adequate evidence to provide specific recommendations about most symptoms that patients may describe in the setting of CIM nor to say whether the symptoms may in fact be attributable to the CIM. Regardless, across the literature reviewed, strain-induced occipital headaches were the symptom most consistently demonstrated to improve with treatment of CIM.
Question 2-2. In patients with asymptomatic CIM without syrinx, is prophylactic surgery indicated to prevent future need for surgery? What is the chance of developing symptoms in the future?
Recommendation: Clinicians should not perform prophylactic surgery on patients with asymptomatic CIM without syrinx. There is a small percentage of patients who develop new or worsening symptoms in the future.
Strength of recommendation: Grade C
Class III Evidence
There were 3 studies identified by the methodology that compared operative and nonoperative treatment of patients with CIM and were thus used in formulating the recommendation. Of note, although the papers may include patients with syrinx, most of the patients were treated nonoperatively in the literature and did not have what was determined to be a clinically significant syrinx. As such, the recommendations are for asymptomatic patients without syrinx and should not be generalized to patients with syrinx.
One study32 identified 226 pediatric patients seen for initial consultation for CIM over a 5-year period, with symptoms and/or with syrinx. Of these patients, 34 had surgery and 192 patients were treated nonoperatively. Of the 34 patients who had surgery, 15 had surgery >6 months after the initial consultation, of which only 5 were delayed because of new symptoms/syrinx (n = 4) or symptom progression (n = 1). In total, authors identified 2 patients with worsening symptoms, 1 patient who had symptoms that failed to improve, and 2 who had worsening syrinx. No patients had surgery >2 years after initial consultation. The authors concluded from their data that patients treated nonoperatively are unlikely to progress, suggesting a benign natural history.
An additional pediatric study33 reviewed 95 patients over a 10-year period. Seventy patients were managed conservatively and 25 had surgery (either dural splitting or duraplasty). They noted a higher percentage of improvement in surgical patients, but 41.7% (20/48) of symptomatic patients treated nonsurgically demonstrated improvements in symptoms. Of the conservatively managed group, 45 showed no change in symptoms or new symptoms if asymptomatic and 5 (7.1%) exhibited worsening symptoms over time. They indicated that their study was consistent with the literature suggesting against prophylactic decompression, as the development of new symptoms or deficits was uncommon.
One additional study17 identified was a mixed population study (pediatric and adults) that reviewed patients evaluated from 2000 to 2011 with long-term follow-up. The population was approximately 30% pediatric (<18 years of age). One-hundred nine patients had surgery with 236 recommended for nonsurgical therapy. Of the 236 nonsurgical patients, 78 were able to be contacted and consented to long-term follow-up questions. Of those, 10 were excluded including 8 that had surgery at an outside institution. Of the 68 remaining patients, they calculated that 73% (50/68) of their patients treated without surgery demonstrated stability or improvement in symptoms with 47.1% (32/68) of those patients showing improvement over an average of 4.9 years. Eighteen patients (26.5%) noted worsening of any symptom with 5 of those patients having improvement in another symptom, suggestive of mixed etiology.
These studies were not limited to asymptomatic patients but still provide class III evidence that most patients that are treated conservatively for CIM remain stable or improve, suggesting a benign natural history.
There were 4 additional studies identified below that, while they did not meet inclusion criteria due to a focus on natural history (and/or do not explicitly address this PICO question), suggest similar conclusions.
Novegno et al34 reviewed patients evaluated for Chiari at their institution from 1988 to 2007. Of a total of 94 children, there were 22 patients with mild or absent clinical symptoms that were followed for ≥3 years (mean 5.9 years). Eleven of the patients were asymptomatic and the other 11 had mild symptoms not felt to warrant intervention. Of the 22 patients, 17 (77.3%) remained asymptomatic or had improvement in the mild symptoms. Five patients had worsening symptoms, of which 2 were mild and were still observed and 3 ultimately had surgery. Of those patients, 2 had endoscopic third ventriculostomy performed for worsening hydrocephalus, suggesting secondary Chiari. The third patient had a Chiari decompression (1 of 22 patients treated directly for Chiari [4.5%]). Based on their data and review of the literature, the authors conclude that conservative treatment is appropriate for asymptomatic and slightly symptomatic patients with Chiari malformation.
Strahle et al35 reviewed the natural history of patients at their institution following a decision to treat conservatively. They included 147 patients who had a CIM diagnosed on magnetic resonance imaging that were not offered surgery and had ≥1 year of follow-up. They had a mean clinical follow-up of 4.6 years and mean imaging follow-up of 3.8 years. One hundred thirty-three of the 147 patients (90.5%) remained asymptomatic or minimally symptomatic. Fourteen patients (9.5%) progressed to surgery with indications including new syrinx or syrinx progression, worsening and refractory headaches, sleep apnea, concern for neurologic decline, and progression of scoliosis. In addition to patients with worsening syrinx, there were 3 patients with resolution of the syrinx as well. They reviewed the data on cerebrospinal fluid (CSF) flow studies on 74 patients with adequate data and noted improvement in CSF flow in 23, no change in 39, and worsening in 12. There were not significant anatomic differences in the patients who later required surgery from those that did not. Based on their data, the authors concluded that the natural history for patients selected for nonoperative management of Chiari is typically benign, with a small percentage of patients having changes.
Benglis et al36 retrospectively reviewed 124 cases of pediatric patients treated nonoperatively and seen over a 10-year period. Eighty-one patients were symptomatic, with 67 felt to have symptoms not typical of Chiari malformation. Of the 14 with symptoms felt typical for Chiari malformation, 9 had symptoms not frequent or severe enough to recommend intervention and 5 were offered surgery. Of the 14 patients with Chiari symptoms, 6 experienced symptom improvement over time, 4 had stable symptoms over time, and 4 had worsening symptoms. No new neurologic deficits were noted among the patients. They concluded that most patients with CIM followed over time do not progress clinically, suggesting a benign natural history.
Another more recent study37 reviewed prospectively collected data on patients with incidentally discovered CIM ≤18 years of age between 2009 and 2019 with at least 12 months of follow-up. They reviewed 218 consecutive patients with a mean follow-up of 40.6 months. Thirty-six patients (16.5%) underwent decompression surgery. Twenty-two of the patients underwent surgery within 6 months of diagnosis, while 14 patients had surgery >6 months after diagnosis. Indications included development of a syrinx (n = 6), syrinx and symptom progression (n = 3), and symptom progression alone (n = 5). Thus, for the patients that did not have surgery within the first 6 months, 7.1% (14/196) progressed to requiring treatment within the review period. The studies that met inclusion criteria, as well as others identified in discussion, support that patients diagnosed with CIM that is not symptomatic enough to warrant intervention generally have a benign natural history, which does not support prophylactic surgery. There is a small percentage of patients that can worsen over time; therefore, clinical and imaging follow-up should be considered on a case-by-case basis and patients can be followed for changes to ensure they are not in a subset that requires treatment in the future.
Question 2-3. In patients with asymptomatic CIM without syrinx, should the patient have any activity restrictions to prevent future harm?
Recommendation: Clinicians should not recommend activity restrictions for patients with asymptomatic CIM without syrinx, as there is no evidence of future harm prevention.
Strength of recommendation: Grade C
Four studies met criteria for inclusion regarding activity restrictions in patients with asymptomatic CIM. Two studies looked at sports participation and risk of injury related to Chiari. Two other studies provide more indirect evidence of potential worsening of symptoms in CIM patients who have a traumatic injury.
Class II Evidence
Strahle et al38 performed a prospective dual site survey study of 503 patients with CIM including 328 sports participants. There was no difference in disease severity with tonsillar ectopia on average 11 mm in both sports participants and sports abstaining cohorts; 74% of all patients had pegged tonsillar morphology and 74% of available CSF flow imaging showed diminished flow. Respondents played a wide variety of high impact sports and over 4641 seasons there were no catastrophic or permanent neurologic injuries.
Class III Evidence
Meehan et al39 performed a single-institution retrospective cohort study over 3 years in 147 patients with an average tonsillar ectopia of 11 mm, again with the majority exhibiting pegged tonsils and crowding at the foramen magnum. Similar results were found including no deaths, coma, or paralysis in 1627 athletic seasons.
In terms of more indirect evidence of worsening with trauma, Wan et al40 performed a single-center retrospective series identifying 85 patients seen with CIM over 21 years. They noted that patients can have onset of symptoms after a minor injury. Freeman et al41 reviewed the cervical spine magnetic resonance imaging scans of 1200 individuals with neck pain, 600 with a history of a whiplash type injury and 600 without a traumatic injury. They noted a statistically significant increase in cerebellar ectopia in patients with a history of whiplash type injury, suggesting a connection between the trauma and worsening cerebellar ectopia or symptoms.
Although there is potentially indirect evidence of possible worsening with trauma, the 2 studies that directly address need for restrictions, 1 prospective38 and 1 retrospective survey study,39 similarly found no poor outcomes in pediatric patients with CIM related to sports participation. Taken together, the studies recommend against activity restrictions for these patients.
It is important to note that this discussion is relevant to patients with asymptomatic CIM and does not apply to patients with symptomatic CIM or with significant skull base abnormalities such as basilar invagination. Overall, although there is some evidence of patients developing symptoms after trauma, there were no patients presented with known Chiari that were followed and worsened. As such, there is no direct evidence of worsening based on activity. Despite a potential increased theoretical risk relative to the general population, the reviewed literature demonstrated no significant injuries in patients with known asymptomatic Chiari from sports participation. The literature supports appropriate counseling of potential risks and individualized discussion with patients and family members but does not provide evidence to support routinely restricting the activity of patients with asymptomatic CIM.
Question 2-4. In patients with CIM, should sleep or swallow studies be routinely performed to evaluate for sleep apnea or dysphagia?
Recommendation: There is insufficient evidence to support routine sleep and swallow studies in patients with CIM without sleep or swallow symptoms.
Strength of recommendation: Grade insufficient
Class III Evidence
There were no studies that met inclusion criteria to address routine swallow studies with CIM. There were 2 retrospective single-institution studies identified that investigated sleep disordered breathing (SDB) in patients with CIM.
Amin et al42 performed a retrospective review on patients with CIM who underwent baseline polysomnography (PSG). They identified 68 children. They noted a 49% prevalence of SDB based on the apnea-hypopnea index, with obstructive apnea being the predominant type of SDB. Tonsillar descent did not predict the presence of SDB in their cohort but was significantly correlated with the obstructive apnea-hypopnea index but not the central apnea index. Using a cutoff of 20 mm herniation, there was a statistically significant association of the level of tonsillar descent with the presence of obstructive sleep apnea.
Khatwa et al43 reviewed 22 children with CIM and SDB on PSG. Seventeen patients had known Chiari before the PSG study, including 5 patients that were asymptomatic. Five patients had symptoms that led to the sleep study prior to the diagnosis of Chiari. They found that the extent of herniation was significantly greater in patients with SDB than those with normal PSG (16.0 vs 8.2 mm mean descent). There were 4 patients with preoperative testing that underwent decompression. All patients showed improvement, but 1 still required treatment for residual sleep apnea. In addition to the demonstration of improvement, they conclude that imaging parameters may correlate with the presence of SDB.
One additional study was excluded based on not having enough patients but did look at benefits of treatment of Chiari in patients with sleep apnea. Addo et al44 showed significant benefit in measured numbers of central sleep apnea postdecompression in 5 patients with known sleep apnea in the setting of CIM and syndromic synostosis, indicating that treating the Chiari malformation can improve SDB.
SDB and swallowing dysfunction may be present in patients with CIM. When these symptoms are present, many providers obtain sleep and swallow studies for further evaluation. The literature reviewed does support the possibility of improvement in SDB after decompression surgery with patients across studies demonstrating improvement. The reviewed literature also suggests that breathing changes may be more prevalent with increasing tonsillar descent on imaging, especially with very significant tonsillar descent (>20 mm). Thus, further clinical workup may be appropriate on an individual basis for many patients with CIM based on clinical suspicion or significant imaging findings. There is no evidence in the literature to support the need for sleep or swallow studies in the routine evaluation of all patients with CIM.
Question 2-5. In patients with CIM, should siblings or first-degree relatives be screened for CIM?
Recommendation: Clinicians should not routinely screen asymptomatic siblings or first-degree relatives of patients with CIM.
Strength of recommendation: Grade C
Class III Evidence
There are different data that suggest a familial association of CIM. Case series identify families with multiple members with CIM,45 suggesting an inherited basis. There are studies that look at the presence of CIM in patients with other genetic disorders, such as neurofibromatosis type 1.46 There is also ongoing research investigating the underlying genetic basis of CIM. One recent study47 performed whole-exome screening on 51 unrelated surgical patients with CIM. They also tested the parents of the patients. They identified multiple genetic variants, including a high number in chromatin-remodeling genes. They highlighted that CIM is likely underdiagnosed in the population as 21 patients had a parent with the same genetic variant, who on imaging were documented to have a CIM despite only 4 of the 51 patients having a known family member with CIM before enrollment in the study.
Based on the data, it seems likely that the family members of patients with CIM are more likely to have the diagnosis than the general population. There is not sufficient evidence to determine a relative risk, however. Although the evidence does support an increased likelihood of having the diagnosis, there is no evidence of a benefit to screening asymptomatic family members or first-degree relatives. Question 2-2 reviews the data on the role of prophylactic surgery on asymptomatic patients and there was no evidence to support prophylactic surgery in those patients. Therefore, without evidence that patients would benefit from intervention, there is no support for routine screening of asymptomatic siblings or first-degree relatives of patients with CIM. It is important to indicate that the guideline recommendation is based on family members being asymptomatic. If family members are symptomatic with concerns for symptoms possibly related to CIM, providers may wish to pursue further evaluation because of the apparent underlying familial predisposition.
Future Research
Review of the literature for the guidelines highlights the lack of Class I evidence to make strong recommendations. It highlights the need for multicenter prospective data collections regarding symptoms and natural history as well as surgical studies. The randomized posterior fossa decompression versus posterior fossa decompression with duraplasty study recently completed will provide some additional data for the pediatric population, but more such studies will be needed to better clarify the optimal management for patients with CIM.
Future studies and collaborative efforts may offer more insight to improve our management approach. Patient-centered studies evaluating patient-reported outcomes may be helpful to inform future clinical decision making and recommendations. It is imperative to explore these questions are help improve care of our patients with CIM and syringomyelia.
CONCLUSIONS
There was primarily Class III evidence for these questions as well as differences across studies, highlighting the need for better data to generate stronger conclusions and recommendations. As such, the guidelines are worded to provide guidance but allow for practitioners to assess and treat patients on an individual basis, based on individual symptoms and characteristics.
Conflicts of Interest
All Guideline Task Force members were required to disclose all potential COIs prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Review Committee. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination and participation on the task force. The CNS Guidelines Committee and Guideline Task Force Chair may approve nominations of task force members with possible conflicts and restrict the writing, reviewing, and/or voting privileges of that person to topics that are unrelated to the possible COIs. See Appendix V for a complete list of disclosures.
Disclosure of Funding
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document.
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a physician volunteer task force as an educational tool that reflects the current state of knowledge at the time of completion. Each chapter is designed to provide an accurate review of the subject matter covered. This guideline is disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient’s physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Acknowledgments
The guidelines task force would like to acknowledge the CNS Guidelines Committee for their contributions throughout the development of the guideline, the AANS/CNS Joint Guidelines Review Committee, as well as the contributions of Kirsten Aquino, contracted project manager for the CNS, Trish Rehring, MPH, Associate Director for Evidence-Based Practice Initiatives for the CNS, and Janet Waters, MLS, BSN, RN, for assistance with the literature searches. The guidelines task force would also like to acknowledge the contributions of Dorothy Poppe, Kaitlyn Esposito, MPH and Mary Poppe, as well as the Bobby Jones Chiari and Syringomyelia Foundation for serving as patient advocates on this guideline task force. Throughout the review process, the reviewers and authors were blinded from one another. At this time the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Jennifer Sweet, MD, Andrew Carlson, MD, MS, Matthew Reynolds, MD, PhD, Alexandra D. Beier, D.O., FACOS, FAAP, Jonathan Pindrik, MD and Patti Raksin, MD.
REFERENCES
1. Aitken LA, Lindan CE, Sidney S, et al. Chiari type I malformation in a pediatric population. Pediatric Neurology. 2009;40(6):449-454.
2. Meadows J, Kraut M, Guarnieri M, Haroun RI, Carson BS. Asymptomatic Chiari type I malformations identified on magnetic resonance imaging. Journal of neurosurgery. 2000;92(6):920-926.
3. Morris Z, Whiteley WN, Longstreth Jr WT, et al. Incidental findings on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ (Online). 2009;339(7720):547-550.
4. Strahle J, Muraszko KM, Kapurch J, Bapuraj JR, Garton HJ, Maher CO. Chiari malformation type I and syrinx in children undergoing magnetic resonance imaging. Journal of neurosurgery Pediatrics. 2011;8(2):205-213.
5. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. New England Journal of Medicine. 2007;357(18):1821-1828.
6. Ransohoff DF, M. Pignone, and H.C. Sox, . How to decide whether a clinical practice guideline is trustworthy. . JAMA. 2013;309(2):139-140.
7. Dones J, De Jesús O, Colen CB, Toledo MM, Delgado M. Clinical outcomes in patients with Chiari I malformation: a review of 27 cases. Surgical neurology. 2003;60(2):142-147; discussion 147-148.
8. Grangeon L, Puy L, Gilard V, et al. Predictive factors of headache resolution after Chiari type 1 malformation surgery. World neurosurgery. 2017;110:e60-e66.
9. Massimi L, Frassanito P, Chieffo D, Tamburrini G, Caldarelli M. Bony decompression for Chiari malformation type I: long-term follow-Up. Acta neurochirurgica Supplement. 2019;125:119-124.
10. Ma J, You C, Chen H, Huang S, Ieong C. Cerebellar tonsillectomy with suboccipital decompression and duraplasty by small incision for Chiari I malformation (with syringomyelia): long term follow-up of 76 surgically treated cases. Turkish neurosurgery. 2012;22(3):274-279.
11. Straus D, Foster K, Zimmerman F, Frim D. Chiari drop attacks: surgical decompression and the role of tilt table testing. Pediatric neurosurgery. 2009;45(5):384-389.
12. Kumar A, Patni AH, Charbel F. The Chiari I malformation and the neurotologist. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2002;23(5):727-735.
13. Beretta E, Vetrano IG, Curone M, et al. Chiari malformation-related headache: outcome after surgical treatment. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2017;38(Suppl 1):95-98.
14. Spena G, Bernucci C, Garbossa D, Valfrè W, Versari P. Clinical and radiological outcome of craniocervical osteo-dural decompression for Chiari I-associated syringomyelia. Neurosurgical review. 2010;33(3):297-303; discussion 303-294.
15. Kumar A, Ghosh SN, Sadique SI. Clinicoradiological study of adult Chiari malformation type 1 patients with emphasis on cerebrospinal fluid peak flow velocity at foramen magnum level. Neurology India. 2019;67(3):744-748.
16. Jia C, Li H, Wu J, et al. Comparison decompression by duraplasty or cerebellar tonsillectomy for Chiari malformation-I complicated with syringomyelia. Clinical neurology and neurosurgery. 2018;176:1-7.
17. Chavez A, Roguski M, Killeen A, Heilman C, Hwang S. Comparison of operative and non-operative outcomes based on surgical selection criteria for patients with Chiari I malformations. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2014;21(12):2201-2206.
18. Parker SL, Godil SS, Zuckerman SL, et al. Comprehensive assessment of 1-year outcomes and determination of minimum clinically important difference in pain, disability, and quality of life after suboccipital decompression for Chiari malformation I in adults. Neurosurgery. 2013;73(4):569-581; discussion 581.
19. McGirt MJ, Nimjee SM, Floyd J, Bulsara KR, George TM. Correlation of cerebrospinal fluid flow dynamics and headache in Chiari I malformation. Neurosurgery. 2005;56(4):716-721; discussion 716-721.
20. Raza-Knight S, Mankad K, Prabhakar P, Thompson D. Headache outcomes in children undergoing foramen magnum decompression for Chiari I malformation. Archives of disease in childhood. 2017;102(3):238-243.
21. Hayhurst C, Richards O, Zaki H, Findlay G, Pigott TJ. Hindbrain decompression for Chiari-syringomyelia complex: an outcome analysis comparing surgical techniques. British journal of neurosurgery. 2008;22(1):86-91.
22. Tisell M, Wallskog J, Linde M. Long-term outcome after surgery for Chiari I malformation. Acta neurologica Scandinavica. 2009;120(5):295-299.
23. De Vlieger J, Dejaegher J, Van Calenbergh F. Multidimensional, patient-reported outcome after posterior fossa decompression in 79 patients with Chiari malformation type I. Surgical neurology international. 2020;10:242.
24. Liu Z, Hao Z, Hu S, Zhao Y, Li M. Predictive value of posterior cranial fossa morphology in the decompression of Chiari malformation type I: A retrospective observational study. Medicine. 2019;98(19):e15533.
25. Caldarelli M, Novegno F, Vassimi L, Romani R, Tamburrini G, Di Rocco C. The role of limited posterior fossa craniectomy in the surgical treatment of Chiari malformation type I: experience with a pediatric series. Journal of neurosurgery. 2007;106(3 Suppl):187-195.
26. McGirt MJ, Attenello FJ, Atiba A, et al. Symptom recurrence after suboccipital decompression for pediatric Chiari I malformation: analysis of 256 consecutive cases. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2008;24(11):1333-1339.
27. Albert GW, Menezes AH, Hansen DR, Greenlee JD, Weinstein SL. Chiari malformation type I in children younger than age 6 years: presentation and surgical outcome. Journal of neurosurgery Pediatrics. 2010;5(6):554-561.
28. Yates C, Campbell R, Wood M, Chaseling R, Tollesson G, Ma N. Chiari 1 malformation: age-based outcomes in a paediatric surgical cohort. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2020;36(11):2807-2814.
29. Greenlee JD, Donovan KA, Hasan DM, Menezes AH. Chiari I malformation in the very young child: the spectrum of presentations and experience in 31 children under age 6 years. Pediatrics. 2002;110(6):1212-1219.
30. Grahovac G, Pundy T, Tomita T. Chiari type I malformation of infants and toddlers. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2018;34(6):1169-1176.
31. Almotairi FS, Hellström P, Skoglund T, Nilsson Å L, Tisell M. Chiari I malformation-neuropsychological functions and quality of life. Acta neurochirurgica. 2019;162(7):1575-1582.
32. Carey M, Fuell W, Harkey T, Albert GW. Natural history of Chiari I malformation in children: a retrospective analysis. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2020.
33. Pomeraniec IJ, Ksendzovsky A, Awad AJ, Fezeu F, Jane JA, Jr. Natural and surgical history of Chiari malformation type I in the pediatric population. Journal of neurosurgery Pediatrics. 2015;17(3):343-352.
34. Novegno F, Caldarelli M, Massa A, et al. The natural history of the Chiari type I anomaly. Journal of neurosurgery Pediatrics. 2008;2(3):179-187.
35. Strahle J, Muraszko KM, Kapurch J, Bapuraj JR, Garton HJ, Maher CO. Natural history of Chiari malformation type I following decision for conservative treatment. Journal of neurosurgery Pediatrics. 2011;8(2):214-221.
36. Benglis D, Jr., Covington D, Bhatia R, et al. Outcomes in pediatric patients with Chiari malformation Type I followed up without surgery. Journal of neurosurgery Pediatrics. 2011;7(4):375-379.
37. Davidson L, Phan TN, Myseros JS, et al. Long-term outcomes for children with an incidentally discovered Chiari malformation type 1: what is the clinical significance? Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2020.
38. Strahle J, Geh N, Selzer BJ, et al. Sports participation with Chiari I malformation. Journal of neurosurgery Pediatrics. 2015;17(4):403-409.
39. Meehan WP, 3rd, Jordaan M, Prabhu SP, Carew L, Mannix RC, Proctor MR. Risk of athletes with Chiari malformations suffering catastrophic injuries during sports participation is low. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2014;25(2):133-137.
40. Wan MJ, Nomura H, Tator CH. Conversion to symptomatic Chiari I malformation after minor head or neck trauma. Neurosurgery. 2008;63(4):748-753; discussion 753.
41. Freeman MD, Rosa S, Harshfield D, et al. A case-control study of cerebellar tonsillar ectopia (Chiari) and head/neck trauma (whiplash). Brain injury. 2010;24(7-8):988-994.
42. Amin R, Sayal P, Sayal A, et al. The association between sleep-disordered breathing and magnetic resonance imaging findings in a pediatric cohort with Chiari 1 malformation. Canadian respiratory journal. 2014;22(1):31-36.
43. Khatwa U, Ramgopal S, Mylavarapu A, et al. MRI findings and sleep apnea in children with Chiari I malformation. Pediatric neurology. 2013;48(4):299-307.
44. Addo NK, Javadpour S, Kandasamy J, Sillifant P, May P, Sinha A. Central sleep apnea and associated Chiari malformation in children with syndromic craniosynostosis: treatment and outcome data from a supraregional national craniofacial center. Journal of neurosurgery Pediatrics. 2012;11(3):296-301.
45. Milhorat TH, Chou MW, Trinidad EM, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44(5):1005-1017.
46. Tubbs RS, Rutledge SL, Kosentka A, Bartolucci AA, Oakes WJ. Chiari I malformation and neurofibromatosis type 1. Pediatric neurology. 2004;30(4):278-280.
47. Provenzano A, La Barbera A, Scagnet M, et al. Chiari 1 malformation and exome sequencing in 51 trios: the emerging role of rare missense variants in chromatin-remodeling genes. Human genetics. 2020.
Appendix I. Literature searches
Literature searches can be found in Chapter 1, Appendix I.
Appendix II. Rating evidence quality
Classification of Evidence on Therapeutic Effectiveness and Levels of Recommendation
Class I evidence
Level I (or A) recommendation |
Evidence from one or more well-designed, randomized controlled clinical trial, including overviews of such trials |
Class II evidence
Level II (or B) recommendation |
Evidence from one or more well-designed comparative clinical studies, such as non-randomized cohort studies, case-control studies, and other comparable studies, including less well-designed randomized controlled trials |
Class III evidence
Level III (or C) recommendation |
Evidence from case series, comparative studies with historical controls, case reports, and expert opinion, as well as significantly flawed randomized controlled trials |
Classification of Evidence on Prognosis and Levels of Recommendation
Class I evidence
Level I (or A) recommendation |
All 5 technical criteria above are satisfied |
Class II evidence
Level II (or B) recommendation |
Four of 5 technical criteria are satisfied |
Class III evidence
Level III (or C) recommendation |
Everything else |
Classification of Evidence on Diagnosis and Levels of Recommendation
Class I evidence
Level I (or A) recommendation |
Evidence provided by one or more well-designed clinical studies of a diverse population using a “gold standard” reference test in a blinded evaluation appropriate for the diagnostic applications and enabling the assessment of sensitivity, specificity, positive and negative predictive values, and, where applicable, likelihood ratios |
Class II evidence
Level II (or B) recommendation |
Evidence provided by one or more well-designed clinical studies of a restricted population using a “gold standard” reference test in a blinded evaluation appropriate for the diagnostic applications and enabling the assessment of sensitivity, specificity, positive and negative predictive values, and, where applicable, likelihood ratios |
Class III evidence
Level III (or C) recommendation |
Evidence provided by expert opinion or studies that do not meet the criteria for the delineation of sensitivity, specificity, positive and negative predictive values, and, where applicable, likelihood ratios |
Classification of Evidence on Clinical Assessment and Levels of Recommendation
Class I evidence
Level I (or A) recommendation |
Evidence provided by one or more well-designed clinical studies in which interobserver and/or intraobserver reliability is represented by a kappa statistic >0.60 |
Class II evidence
Level II (or B) recommendation |
Evidence provided by one or more well-designed clinical studies in which interobserver and/or intraobserver reliability is represented by a kappa statistic >0.40 |
Class III evidence
Level III (or C) recommendation |
Evidence provided by one or more well-designed clinical studies in which interobserver and/or intraobserver reliability is represented by by a kappa statistic <0.40 |
Appendix III. PRISMA flowchart
Question 2-1.
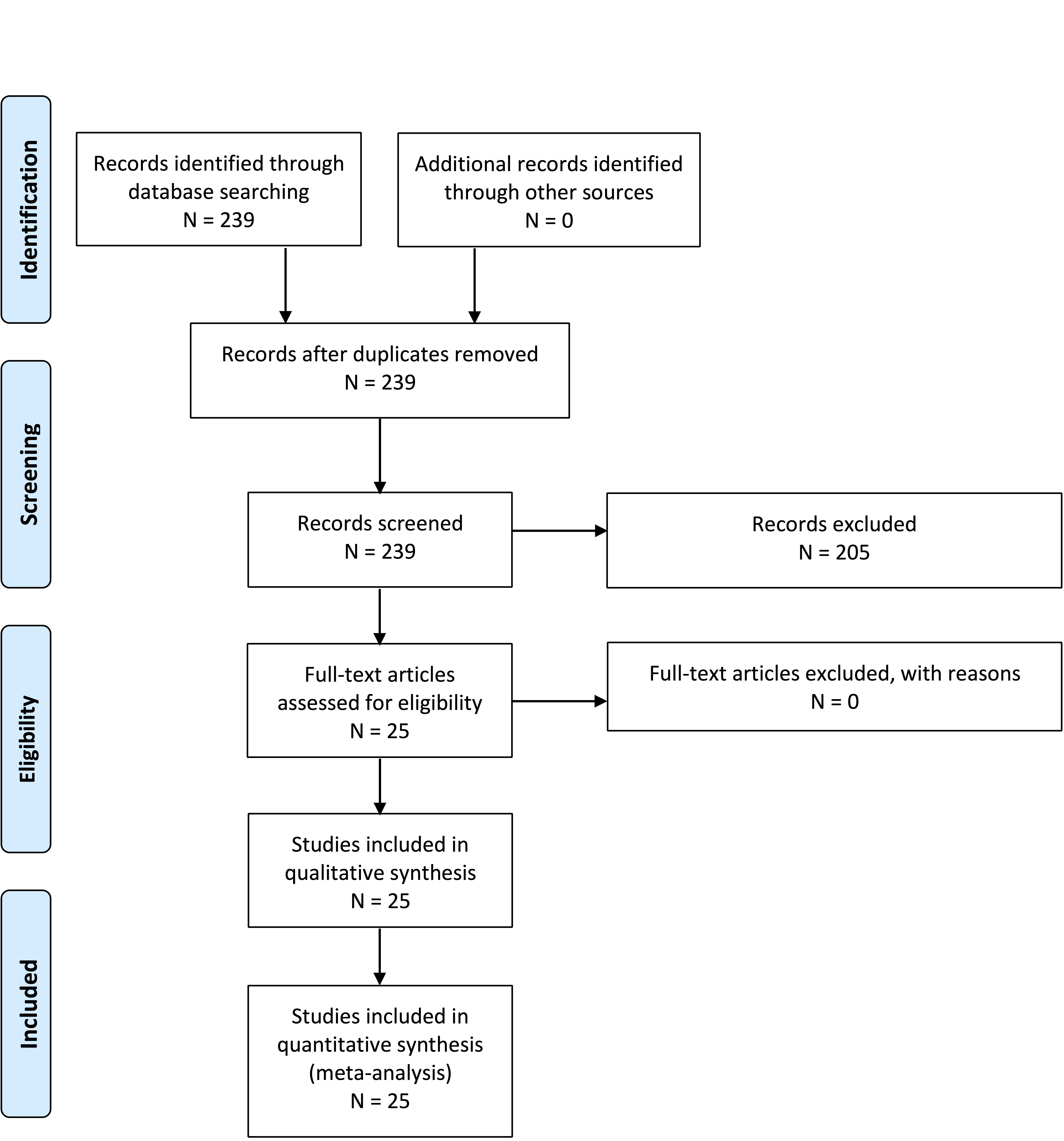
Question 2-2.
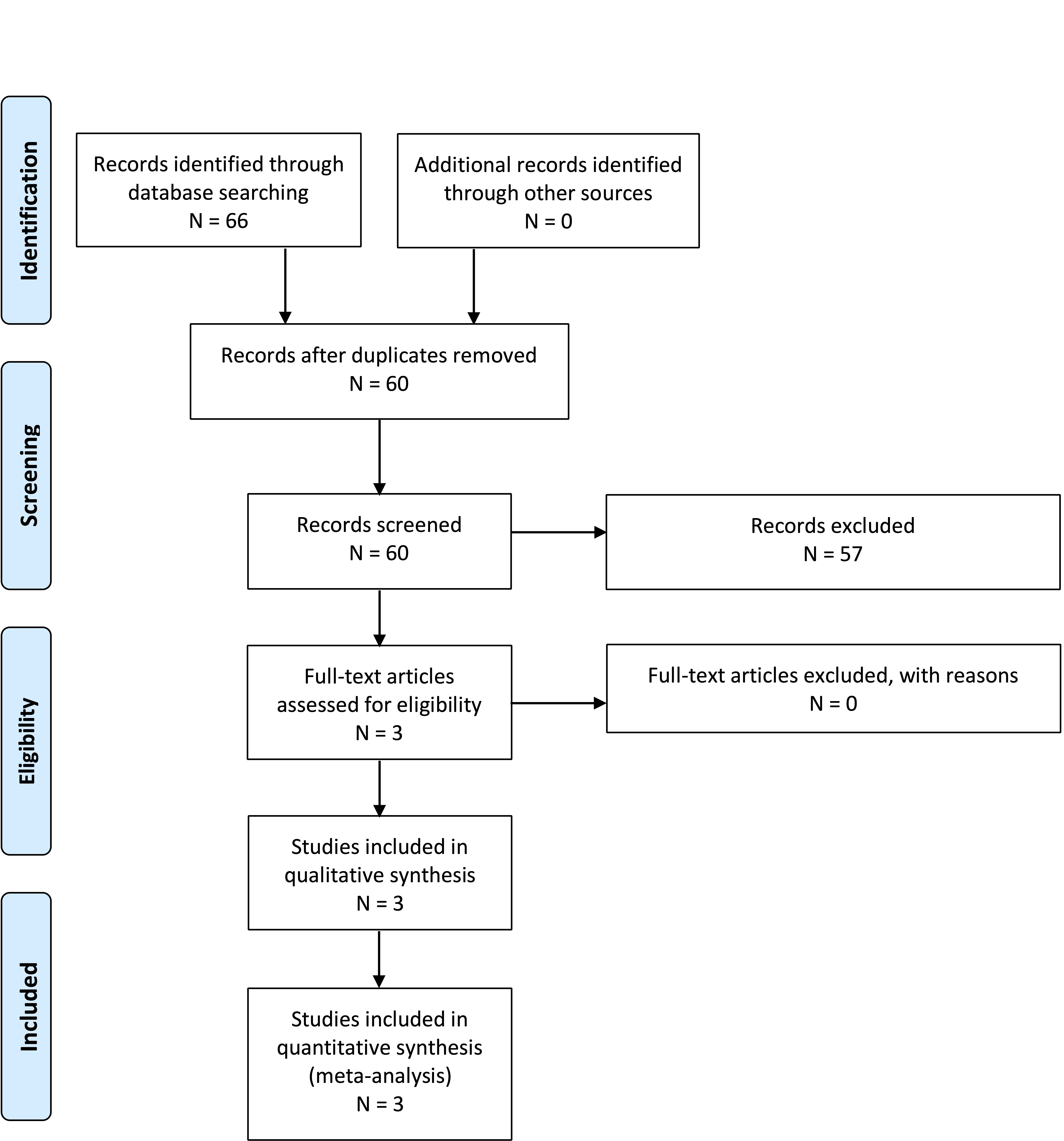
Question 2-3.
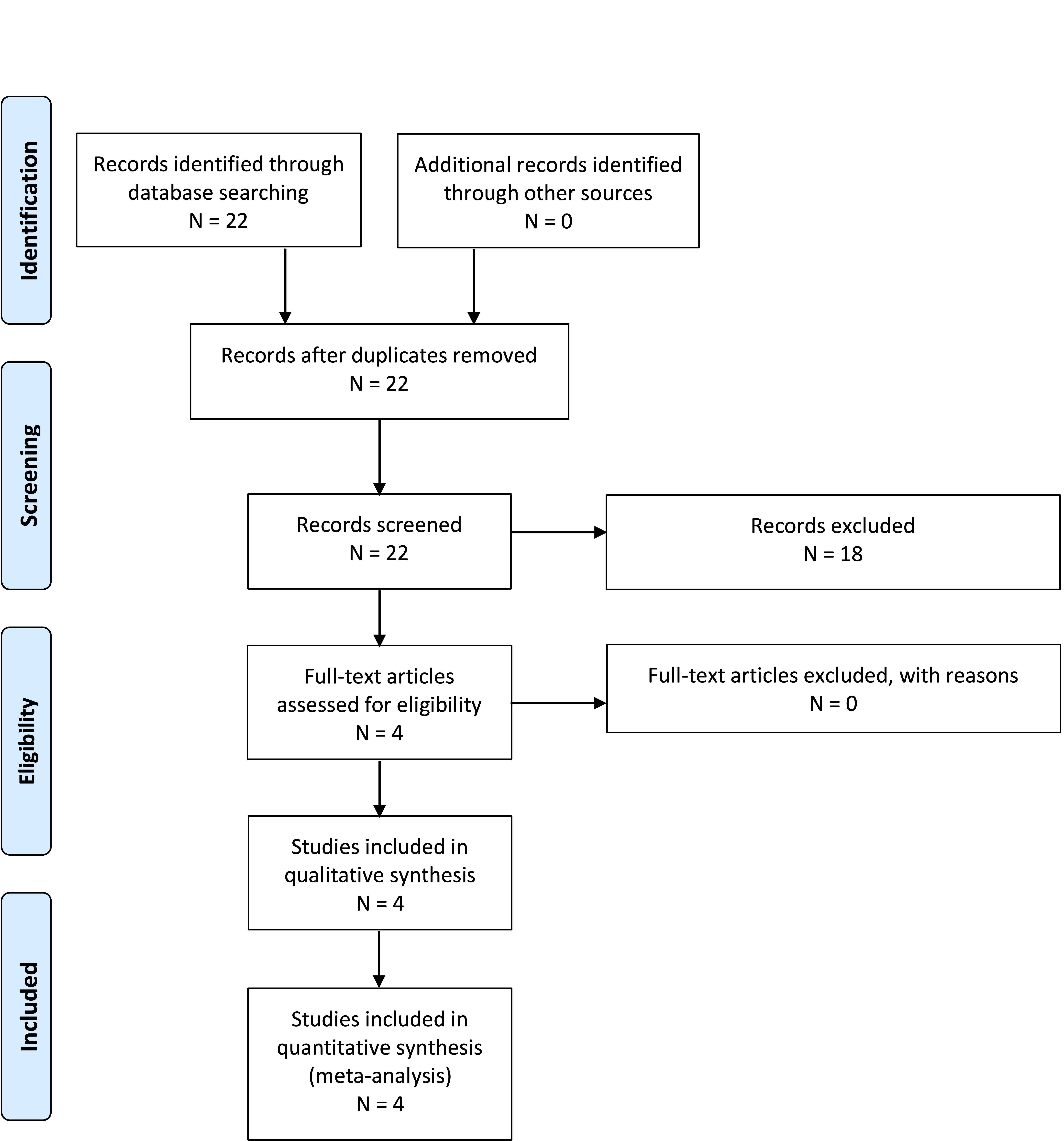
Question 2-4.
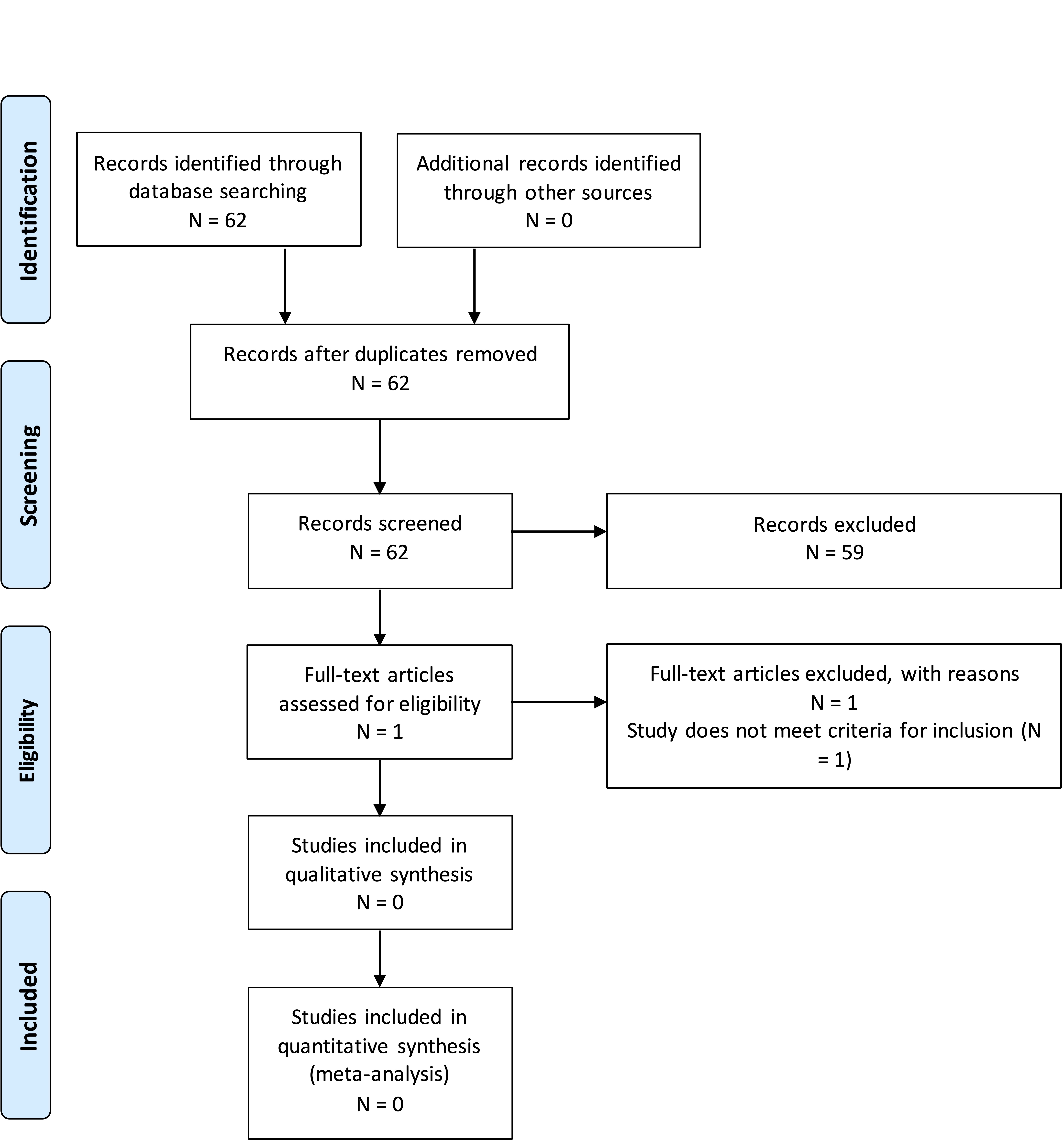
Question 2-5.
Appendix IV. Evidence Tables
| PICO |
Author, Year |
Literature Type |
Study Design |
Class of Evidence |
Author Conclusions |
| 1 |
Massimi et al, 20199 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 42 children with CIM and symptoms including headache (81%), neck pain (40%), vertigo (40%), ataxia (26%), and upper and lower extremity paraesthesia (26%). Resolution and significant improvement of preoperative symptoms was achieved in 36.5% and 21.5% after bone-only decompression, respectively |
| 1 |
Ma et al, 201210 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 76 pediatric and adult patients with neurologic symptoms and combined structural diagnoses of CIM and syringomyelia, or either alone. In this mixed surgical population 80% of patients improved, with 16% stabilized and 4% worsened; 98% of patients with syringomyelia improved or stabilized while expansion occurred in 2% |
| 1 |
Yates et al, 202028 |
Patient assessment |
Retrospective comparative |
III |
Retrospective review for 65 pediatric patients with CIM who underwent PFD. Important differences were found between very young patients aged ≤6 and children ages 7-18 years; very young children scored significantly lower on the CCOS, returned to the operating room more frequently for revision surgery, and presented differently with more common oropharyngeal and motoric symptomatology |
| 1 |
Straus et al 200911 |
Diagnostic test |
Retrospective case series |
III |
Retrospective review of a subpopulation of patient with CIM and drop attacks with a negative cardiac workup for syncope, tilt table test, and surgical decompression. Ten patients met inclusion criteria and half had a positive tilt test. Following PFD, 7/10 patients improved, and the tilt test accuracy was determined to be 40% with a recommendation that it has poor value predicting clinical response in Chiari drop attack patients |
| 1 |
Kumar et al, 200212 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 77 patients with CIM who were evaluated for dizziness, hearing loss, and tinnitus at a vestibular laboratory. Following a battery of vestibular tests, surgical decompression was performed on 33 patients. Surgery-dependent improvement in SNHL and vestibular dysfunction was not strictly reported but the conclusions are that this battery may help guide neurosurgeons for a subset of patients |
| 1 |
Greenlee et al, 200229 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 31 children <6 years of age with CIM and symptoms including oropharyngeal function (35%), scoliosis (23%), headache or neck pain (23%), sensory disturbance (6%), weakness (3%), and other (10%). Preoperative symptoms resolved in 31%, improved in 42%, and were unchanged in 27% after PFD |
| 1 |
Almotairi et al, 202031 |
Patient assessment |
Prospective case series |
III |
Prospectively reviewed 11 adult patients with CIM who underwent pre- and postoperative neuropsychiatric and QOL testing. CIM patients reported lower life satisfaction compared with normal control subjects before and after surgery; however, visual analogue and descriptive responses indicated that their QOL was significantly improved after surgery. Surgery also improved responses in dimensions testing in executive functioning, verbal learning, psychomotor speed, and color naming speed |
| 1 |
Albert et al, 201027 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 39 CIM patients aged <6 years identifying age-dependent symptomatology upon presentation. Early recognition and treatment by different surgical approaches lead to good outcomes, including complete resolution of gastroesophageal reflux, and significant amelioration of headache |
| 1 |
Beretta et al, 201713 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 135 patients assessing headache improvement after Chiari decompression, noting improvement in 93% with typical headache and 85% of patients with atypical headache |
| 1 |
Spena et al, 201014 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 39 CIM patients who presented with variable symptoms. Headache symptoms improved in 80% of patients, whereas neuropathic pain or motor weakness responded less frequently to treatment |
| 1 |
Dones et al, 20037 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 27 patients treated for CIM over a 9-year period with variable improvement in symptoms. Some differences in symptoms that improved pending presence of syrinx |
| 1 |
Kumar et al, 201915 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 30 CIM patients postoperatively for clinical features and peak flow velocity. Overall, 8 (25.6%) of the 32 patients had complete resolution of their symptoms, 18 (56.25%) patients reported partial resolution, and 6 reported no improvement in their symptoms. Headaches responded the best with 16 (88.9%) patients noted some improvement in their headache; 6 had complete resolution, 5 had decreased frequency, 2 were able to control their headache with over-the counter analgesics, and 4 (including 1 of the patients with decreased headache frequency) had resolution of some, but not all, headache subtypes. Other symptoms that were alleviated postoperatively included upper and lower extremity sensory changes (n = 7), neck pain (n = 4), dizziness/vertigo (n = 5), visual symptoms (n = 1), and dysphagia (n = 2). Two patients did not report any improvement in symptoms within 1 year of follow-up. Patients who did not report an immediate clinical improvement continued to experience headaches (n = 2), neck pain/stiffness (n = 2), dizziness (n = 1), and photophobia (n = 1). No patient had a worsening of symptomatology following surgery |
| 1 |
Jia et al, 201916 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 115 adult CIM patients underwent PFD or PFDD with tonsillar resection. Symptoms were grouped into pain, dysesthesias, motor weakness, and gait ataxia. Overall, symptoms were reported improved in 83-88%, unchanged in 9-13%, and worse in 3%. Pain improved the most and there were not significant differences between procedures |
| 1 |
Chavez et al, 201417 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 177 CIM patients, 109 treated surgically and 68 conservatively. Risk factors for clinical improvement were identified and a propensity score defined. The propensity score-adjusted odds for overall improvement was 16.5 times higher for improvement with surgery than patients managed conservatively. Cough headache, migraine or other headache, paresthesia, and ataxia were most highly predictive |
| 1 |
Parker et al, 201318 |
Patient assessment |
Prospective case series |
III |
One-year longitudinal cohort study of 50 CIM patients at a single institution. Headache severity improved in 37 patients (74%), remained the same in 11 (22%), and worsened in 2 (4%).Twenty patients (40%) presented with syringomyelia, 19/20 (95%) had postoperative MRI; 12/19 patients (63%; 4 cervical, 8 thoracic) demonstrated improvement in syrinx size, whereas the remaining 7 patients (37%; 3 cervical, 4 thoracic) showed no change in syringomyelia. Twelve patients (60%) had improved myelopathy. Baseline ventriculomegaly improved in 1 of 3 patients (33.3%) |
| 1 |
McGirt et al, 200519 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 38 patients with headache alone and CIM. Seventeen patients underwent surgical decompression with 7 reporting frontal and 10 reporting occipital headaches. Radiographic CSF obstruction at the foramen magnum was present in 2/7 frontal headaches and 10/10 occipital headache patients. At follow-up 12 months after surgery, 4 (57%) of 7 patients with frontal headaches experienced recurrent headaches versus none (0%) of 10 patients originally presenting with occipital headaches. In the frontal headache group, the 2 patients with obstructed CSF flow had no headache recurrence at follow-up. Furthermore, decompressive treatment failed in 4 (80%) of the 5 patients with nonobstructed flow. Regardless of the degree of tonsillar ectopia, occipital headaches were strongly associated with hindbrain CSF flow abnormalities, whereas frontal and generalized headaches were not. Normal magnetic resonance imaging-cine CSF flow in the setting of a Chiari I malformation and frontal headaches alone suggests that frontal headaches are not pathologically or causatively associated with the Chiari I malformation in most patients. Frontal headaches with obstructed flow may respond to surgery |
| 1 |
Raza-Knight et al, 201720 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 102 CIM patients, 57 (55.9%) presented with headache. Forty-two of 57 (73.7%) were classified as CIM headache, and 32/39 with 3-month follow-up sustained improvement. Duraplasty improved headaches in 32/38 (84.2%) patients receiving such therapy compared with 9/16 (56.3%) treated by bone-only decompression |
| 1 |
Hayhurst et al, 200821 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 96 patients with average follow-up 3.6 years. Postoperative resolution or improvement in symptoms was seen in 75 patients (78%). Drop attacks and headaches were the most likely to respond to hindbrain decompression, showing improvement or resolution in 100% and 92% of cases. Dysaesthetic arm pain and weakness carried the worse prognosis with only 20% having symptom resolution. Sixteen patients had only bony decompression leaving the dura intact. In 8 patients (66%), headaches resolved following bony decompression alone but unchanged in 25% of cases. Dysaesthetic pain and weakness were unchanged in 60%. Restoration of CSF flow dynamics at the foramen magnum by surgical decompression does not consistently result in resolution of symptoms in all patients |
| 1 |
Tisell et al, 200922 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 24 consecutive patients who were contacted about long-term follow-up after Chiari decompression. Seventy-five percent noted an improvement in headache, and 88% noted an improvement in associated neurologic symptoms |
| 1 |
Khatwa et al, 201343 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 22 children with CIM who underwent PFD and polysomnography studies. Diagnoses included central sleep apnea (3), obstructive sleep apnea (5), and both obstructive and central sleep apnea (1). Children with sleep-disordered breathing had excessive crowding of the brainstem structures at the foramen magnum and were more likely to have a greater length of herniation compared with those children without sleep-disordered breathing (p = .046). Patients with central sleep apneas received surgical decompression, and their conditions were significantly improved on follow-up polysomnography |
| 1 |
De Vlieger et al, 201923 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 79 CIM patients who were interviewed about symptom improvement following surgery. Fifty-four patients (68%) reported at least some improvement, 46 (58%) important improvement, 13 (16%) worsening, and 12 stabilization (15%). Any improvement as well as important improvement were significantly more often reported in the nonsyringomyelia group (85% vs 57%, p = .01 and 76% vs 46%, p = .01, respectively). Forty-five of 59 (76%) patients with headaches reported some improvement with 4 (7%) worsening. Sixty-two patients (78%) were satisfied or very satisfied with the results of surgery and 8 (11%) were unsatisfied or very unsatisfied. Up to 71 patients (90%) would consent to surgery again |
| 1 |
Grangeon et al, 20188 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 49 CM1 patients and preoperative headaches. Clinical predictors for patients achieving >50% decrease in headache days included duration <5 min, occipital location, associated with Valsalva maneuver, severe intensity, and greater number of headaches per month; there were no predictive radiological factors. Postoperative improvement was inversely correlated with the Chiari severity index |
| 1 |
Liu et al 201924 |
Patient assessment |
Retrospective Case series |
III |
Retrospective review of 39 patients following PFD. Overall, 24 (61.5%) showed improvement and 15 (38.5%) showed no improvement. Symptoms of motor weakness, lower limb symptoms, muscular atrophy, dizziness, and gait instability were not likely to improve, whereas headache and pain symptoms were likely to improve |
| 1 |
Caldarelli et al, 200725 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 30 CIM pediatric patients who underwent bone-only PFD, although 11 patients also had serial incision of the outer layer of dura. The most frequent symptoms and signs were head and/or neck pain (56.7%), followed by vertigo (27.7%), upper and lower-extremity weakness (20.0%), and ataxia (20.0%). Improvement or resolution in symptoms was found in all patients without any change in tonsillar position and syrinx reduction in half the cases |
| 1 |
McGirt et al, 200826 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 256 patients over 10 years to identify predictors of persistence of symptoms. 192 (75%) had headaches and 68 (27%) had brainstem or cranial nerve symptoms. Fifty-seven patients (22%) experienced mild to moderate symptom recurrence and this was less likely in patients who were treated with concurrent brainstem or cranial nerve involvement. Vertigo and frontal headache were more likely associated with symptom recurrence, and length of time for headache preceding treatment increased symptom recurrence by 15% per year |
| 2 |
Chavez et al, 201417 |
Patient assessment/therapy |
Retrospective case series |
III |
Retrospective mixed population study for patients evaluated from 2000-2011 with long-term follow-up. The population was approximately 30% pediatric (<18 years of age). One hundred nine patients had surgery with 236 managed nonoperatively. Of those, 78 were able to be contacted and consented to long-term follow-up questions. Of those, 10 were excluded including 8 that had surgery at an outside institution. Of the 68 remaining patients, they calculated that 73% (50/68) of their patients treated without surgery demonstrated stability or improvement in symptoms over an average of 4.9 years |
| 2 |
Pomeraniec et al, 201633 |
Patient assessment |
Retrospective case series |
III |
Retrospective review of 95 pediatric CIM patients managed conservatively (70) or with PFD with dural splitting or duraplasty (25). Seventy-five percent of operated patients had significant improvement in clinical symptoms. At the same time, 92.9% of nonoperatively-treated patients did not show progression and 41.7% of those patients showed improvement in symptoms without intervention. There were no differences between the surgical groups |
| 2 |
Carey et al, 202132 |
Patient assessment/therapy |
Retrospective case series |
III |
Retrospective study of 226 pediatric patients seen for initial consultation for Chiari malformation over a 5-year period. The study includes patients with symptoms and patients with syrinx (26). Thirty-four were managed surgically and 192 patients were managed nonoperatively. Fifteen of 34 had surgery greater than 6 months after the initial consultation, 3 due to new symptoms and 1 due to new syrinx. Of those patients, 3 had a Chiari decompression and 1 had a shunt placed. As the study was not limited to asymptomatic patients without, they also had 2 patients with worsening symptoms, 1 patient had symptoms that failed to improve and 2 had worsening syrinx. No patients had surgery >2 years after initial consultation |
| 3 |
Freeman et al, 201041 |
Patient assessment |
Retrospective case control |
III |
Retrospective study comparing scans of 1200 patients with neck pain including 600 with trauma and 600 without trauma. They identified a statistically significantly increased percentage of patients with cerebellar tonsillar ectopia in the group with trauma |
| 3 |
Wan et al, 200840 |
Patient assessment |
Retrospective case series |
III |
Retrospective series of 85 patients over 21 years. The authors note that minor head or neck trauma can precipitate the onset of symptoms in a small number of previously asymptomatic patients with CIM |
| 3 |
Meehan et al 201539 |
Patient assessment |
Retrospective comparative |
III |
Retrospective cohort study over 3 years in 147 patients with an average tonsillar ectopia of 11mm. Most patients exhibited pegged tonsils and crowding at the foramen magnum. Results demonstrated no deaths, coma, or paralysis in 1627 athletic seasons, indicating that the risk of injury is low |
| 3 |
Strahle et al, 201638 |
Patient assessment |
Prospective comparative |
II |
Prospective dual site survey study of 503 patients including 328 sports participants. There was no difference in disease severity in both sports participants and non-sports participants. Respondents played a wide variety of high impact sports and over 4641 seasons there were no catastrophic or permanent neurologic injuries |
| 5 |
Provenzano et al, 202047 |
Patient assessment |
Prospective case series |
III |
Prospective series of patients where they performed whole-exome sequencing of 51 unrelated patients with Chiari malformation. They also tested parents. They noted abnormalities in chromatin remodeling genes. They also found that many patients had parents with the same gene mutation who also were noted to have Chiari malformation on further testing. The authors conclude that in most cases CIM is a dominant, Mendelian inherited trait |
| 5 |
Tubbs et al, 200446 |
Patient assessment |
Retrospective case series |
III |
Retrospective evaluation of 2 groups, 1 with CIM who underwent PFD for symptoms, and the second group is patients observed in clinic with NF-1. 5.4% of 130 patients in group 1 also had NF-1. 8.6% of 198 patients in group 2 with NF-1 also had posterior fossa decompression for symptoms. The authors conclude that there is an association between CIM and NF-1 |
| 5 |
Milhorat et al, 199945 |
Patient assessment |
Prospective case series |
III |
A prospective cohort of 364 symptomatic patients and 50 patients and 50 age- and gender-matched control subjects underwent posterior fossa volumetric analysis using the Cavalieri methodology. Families of 21 patients participated in a study of familial aggregation. There were 275 female and 89 male patients. Age of symptom onset was 24.9 years, and 89 patients cited an immediate history of trauma. Forty-three (12%) patients had a family history of CIM or syringomyelia. The authors conclude that symptoms are associated with a volumetric reduction in CSF while brain volumes may be normal, and that tonsillar ectopia <5 mm does not obviate similar symptomatology. They conclude there is demonstration of familial aggregation that suggests a genetic component |
CCOS, Chicago Chiari Outcome Scale; CIM, Chiari type I malformation; CSF, cerebrospinal fluid; NF-1, neurofibromatosis type 1; PFD, posterior fossa decompression; PFDD, posterior fossa decompression with duraplasty; SNHL, sensorineural hearing loss; QOL, quality of life.
Appendix V. Conflicts of interest
| Name |
Affiliation |
Type of COI |
| Toba Niazi, MD |
Live Like Bella Foundation, Nicklaus Children's Hospital |
Grants/Research Support |
| Laurie Ackerman, MD |
Park Reeves Consortium |
Grants/Research Support |
| David Bauer, MD |
None |
|
| Brandon G. Rocque, MD, MS, FAANS |
None |
|
| Carolyn S. Quinsey, MD |
None |
|
| Eric Jackson, MD |
None |
|
| Jogi V. Pattisapu MD FAAP FAANS |
J&J, Integra |
Consultant |
| Rabia Qaiser, MD |
None |
|
| Cormac O. Maher, MD, FAAP, FACS, FAANS |
None |
|
| Shobhan H. Vachhrajani MD, PhD, FRCSC |
None |
|
| Libby Infinger, MD |
None |
|
| Howard Silberstein, MD |
None |
|
| Sarah Jernigan, MD |
None |
|
| Jeffrey S. Raskin MS MD FAANS FAAP |
None |
|
| Dorothy Poppe |
None |
|
| Kaitlyn Esposito, MPH |
None |
|