Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines for Chiari Malformation: Diagnosis
Download PDF Neurosurgery, 2023
Sponsored by: Congress of Neurological Surgeons (CNS) and the Section on Pediatrics
Endorsed by: The Congress of Neurological Surgeons (CNS), American Association of Neurological Surgeons (AANS), and the Bobby Jones Chiari and Syringomyelia Foundation (Bobby Jones CSF)
Authors:
David F. Bauer, MD, MPH1, Toba Niazi, MD2, Rabia Qaiser, MD3, Libby Kosnik Infinger, MD, MPH4, Shobhan Vachhrajani MD, PhD, FRCSC5, Laurie L Ackerman, MD6, Eric M. Jackson, MD7, Sarah Jernigan, MD, MPH8, Cormac O. Maher, MD, FAAP, FACS, FAANS9, Jogi V. Pattisapu MD FAAP FACS FAANS10, Carolyn Quinsey, MD11, Jeffrey S. Raskin MS MD12, Brandon G. Rocque, MD, MS13, Howard Silberstein, MD14
Departmental and institutional affiliations:
- Department of Neurosurgery, Baylor College of Medicine, Division of Pediatric Neurosurgery, Texas Children’s Hospital, Houston, TX
- Department of Neurological Surgery, Nicklaus Children's Hospital, Miami, FL
- Department of Neurological Surgery, Indiana University School of Medicine, Indianapolis, IN
- Department of Neurosurgery, Medical University of South Carolina (MUSC), Charleston, SC
- Department of Pediatrics, Wright State University Boonshoft School of Medicine, Dayton, OH
- Department of Neurological Surgery, Indiana University Health, Indianapolis, IN
- Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD
- Carolina Neurosurgery & Spine Associates, Charlotte, NC
- Department of Neurosurgery, Stanford Medicine, Palo Alto, CA
- Pediatric Neurosurgery, University of Central Florida College of Medicine, Orlando FL
- Department of Neurosurgery, University of North Carolina Chapel Hill, Chapel Hill, NC
- Department of Neurological Surgery, Northwestern University Feinberg School of Medicine, Chicago, IL
- Division of Pediatric Neurosurgery, Department of Neurosurgery, University of Alabama at Birmingham, Birmingham, AL
- Department of Neurosurgery, University of Rochester School of Medicine and Dentistry, Rochester, NY
Corresponding Author contact information:
David F. Bauer, MD, MPH
Department of Neurosurgery, Baylor College of Medicine
Division of Pediatric Neurosurgery, Texas Children’s Hospital
Houston, TX
dfbauer@texaschildrens.org
No part of this article has been published or submitted for publication elsewhere.
Keywords: Chiari, diagnosis, guideline, systematic review, treatment
Abbreviations: CIM (Chiari I Malformation), MRI (Magnetic Resonance Imaging), CSF (Cerebral spinal fluid)
ABSTRACT
Background: Chiari I malformation (CIM) is characterized by descent of the cerebellar tonsils through the foramen magnum, potentially causing symptoms from compression or obstruction of the flow of cerebrospinal fluid (CSF). Diagnosis and treatment of CIM is varied, and guidelines produced through systematic review may be helpful for clinicians.
Objective: We performed a systematic review of the medical literature to answer specific questions on the diagnosis and treatment of CIM.
Methods: PubMed and Embase were queried between 1946 and January 23, 2021 using the search strategies provided in Appendix I.
Results: The literature search yielded 567 abstracts, of which 151 were selected for full-text review, 109 were then rejected for not meeting the inclusion criteria or for being off-topic, and 42 were included in this systematic review.
Conclusion: Three Grade C recommendations were made based on Level III evidence.
RECOMMENDATIONS
1-1. In patients with CIM diagnosed only with brain or cervical spine MRI, is complete imaging needed to evaluate for clinically relevant pathology such as brain tumor, hydrocephalus, spine syrinx, or tethered spinal cord?
Recommendation: In patients with CIM diagnosed only with brain or cervical spine MRI, further imaging of the brain and spine may be helpful to evaluate for clinically relevant pathology such as hydrocephalus or spine syrinx.
Strength of recommendation: Grade C
Level III evidence
1-2. In patients with CIM, are advanced imaging modalities such as Cine MRI helpful to predict benefit from surgical decompression?
Recommendation: In patients with CIM, advanced imaging modalities such as Cine MRI may or may not predict benefit from surgical decompression.
Strength of recommendation: Grade C
Level III evidence
1-3. In patients with CIM, should flexion and extension radiographs be routinely performed to evaluate for cervical instability? Should preoperative evaluation include any specific evaluation for cranial cervical instability or ventral compression (clivoaxial angle, pB-C2, etc)?
Recommendation: In patients with CIM, measurement of the clivoaxial angle, pB-C2, or C- C2 sagittal vertebral alignment (C-C2SVA) may predict future craniocervical instability and the need for surgical stabilization.
Strength of recommendation: Grade C
Level III evidence
There is insufficient evidence to support the use of flexion-extension films to predict future craniocervical instability in this population.
Strength of recommendation: Grade insufficient
Insufficient evidence
INTRODUCTION
Goals and Rationale
This clinical guideline has been created to improve patient care by outlining the appropriate diagnostic and decision-making processes involved in the treatment of patients with Chiari I malformation (CIM). Diagnosis and treatment of CIM can be challenging because not all patients are symptomatic and many patients do not require surgery. In addition, variations in surgical treatment can make surgical decision-making challenging. This guideline was created as an educational tool to guide qualified physicians through a series of diagnostic and treatment decisions to improve the quality and efficiency of care for patients with CIM.
Objectives
CIM is defined as descent of the cerebellar tonsils ≥3 to 5 mm below the foramen magnum. Based on a definition of a tonsillar position of ≥5 mm below the foramen magnum, imaging studies estimate a prevalence ranging from 0.24% to 2.6% of the population,1–5 including children and adults. Not all patients are symptomatic, and there are various ways to diagnose and treat CIM in the literature. CIM may cause syringomyelia, and some patients with CIM may have craniocervical instability requiring decompression and/or fusion of the craniocervical junction. Symptoms result from blockage of the flow of cerebrospinal fluid (CSF) or from compression of the brainstem or cranial nerves. Treatment may include decompression with or without duraplasty, and intradural tonsil reduction or resection of intradural webs over fourth ventricle outflow have been described. Symptoms reported in the literature are not completely concordant from study to study, and there can be overlap between CIM symptoms and other entities, such as migraine headache, making diagnosis of a symptomatic patient challenging. Because of diagnostic and treatment variability, we initiated the formation of this guideline to systematically review the literature and create evidence-based recommendations for common diagnostic and treatment questions related to CIM. In this guideline, we evaluate the diagnosis of CIM.
METHODOLOGY
The guidelines task force initiated a systematic review of the literature and evidence-based guideline relevant to the diagnosis of patients with CIM. Through objective evaluation of the evidence and transparency in the process of making recommendations, this evidence-based clinical practice guideline was developed for the diagnosis and treatment of patients with CIM. These guidelines are developed for educational purposes to assist practitioners in their clinical decision-making processes. Additional information about the methods used in this systematic review is provided below.
Literature Search
Task force members identified search terms/parameter and a medical librarian implemented the literature search, consistent with the literature search protocol (see Appendix I), using the National Library of Medicine/PubMed database and Embase for the period from 1946 to January 23, 2021, using the search strategies provided in Appendix I.
Inclusion/Exclusion Criteria
Articles were retrieved and included only if they met specific inclusion/exclusion criteria. To reduce bias, these criteria were specified before conducting the literature searches.
Articles that do not meet the following criteria were, for the purposes of this evidence-based clinical practice guideline, excluded. To be included as evidence in the guideline, an article had to be a report of a study that:
- Investigated patients with CIM;
- Studies that enrolled ≥80% of CIM (we included studies with mixed patient populations if they reported results separately for each group/patient population);
- Was a full article report of a clinical study;
- Was not a medical records review, meeting abstract, historical article, editorial, letter, or commentary;
- Appeared in a peer-reviewed publication or a registry report;
- Enrolled a minimum of 10 patients;
- Was of humans;
- Was published in or after 1946;
- Quantitatively presented results;
- Was not an in vitro study;
- Was not a biomechanical study;
- Was not performed on cadavers;
- Was published in English;
- Was not a systematic review, meta-analysis, or guideline developed by others
Systematic reviews or meta-analyses conducted by others or guidelines developed by others were not included as evidence to support this review because of the differences in article inclusion/exclusion criteria specified compared with the criteria specified by the Guidelines Task Force. Although these articles were not included as evidence to support the review, these articles were recalled for full-text review for the Guidelines Task Force to conduct manual searches of the bibliographies.
Assessment for Risk of Bias
The methodological quality of randomized controlled trials and the risk of bias was assessed by using the following 6 criteria: sequence generation, allocation concealment, blinding, incomplete reporting of data, selective reporting of outcomes, and other potential threats to validity. Any bias was discussed and mitigated through clarification in the evidentiary table and changing the grade of the level of evidence, if needed.
1The guideline task force did not include systematic reviews, guidelines, or meta-analyses conducted by others. These documents are developed using different inclusion criteria than those specified in this guideline; therefore, they may include studies that do not meet the inclusion criteria specific to this guideline. In cases where these types of documents’ abstracts suggested relevance to the guideline’s recommendations, the task force searched their bibliographies for additional studies.
Rating Quality of Evidence
The quality of evidence was rated using an evidence hierarchy for each of 4 different study types; therapeutic, prognostic, diagnostic, and decision modeling. These hierarchies are shown in Appendix II: Rating Evidence Quality. Additional information regarding the hierarchy classification of evidence is located at https://www.cns.org/guidelines/guideline-procedures-policies/guideline-development-methodology.
Revision Plans
In accordance with the Institute of Medicine’s standards for developing clinical practice guidelines and criteria specified by the National Guideline Clearinghouse, the task force will monitor related publications after the release of this document and will revise the entire document and/or specific sections “if new evidence shows that a recommended intervention causes previously unknown substantial harm; that a new intervention is significantly superior to a previously recommended intervention from an efficacy or harms perspective; or that a recommendation can be applied to new populations.”1 In addition, the task force will confirm within 5 years from the date of publication that the content reflects current clinical practice and the available technologies for the evaluation and treatment for patients with CIM.
RESULTS
The literature search yielded 567 abstracts. Task force members reviewed all abstracts yielded from the literature search and identified the literature for full-text review and extraction, addressing the clinical questions, in accordance with the literature search protocol (Appendix I). Task force members identified the best research evidence available to answer the targeted clinical questions. When class I, II and or III literature was available to answer specific questions, the task force did not review class IV studies.
The task force selected 151 full-text articles for full-text review. Of these, 109 were rejected for not meeting the inclusion criteria or for being off-topic. Forty-two full-text articles were included in this systematic review (Appendix III).
DISCUSSION
Question 1-1. In patients with CIM diagnosed only with brain or cervical spine MRI, is complete imaging needed to evaluate for clinically relevant pathology, such as brain tumor, hydrocephalus, spine syrinx, or tethered spinal cord?
Recommendation: In patients with CIM diagnosed only with brain or cervical spine MRI, further imaging of the brain and spine may be helpful to evaluate for clinically relevant pathology such as hydrocephalus or spine syrinx.
Strength of recommendation: Grade C
Class III Evidence
There were 29 articles (class III studies) evaluating the relationship between CIM and other diagnoses, including brain lesion, hydrocephalus, scoliosis, syringomyelia, or tethered spinal cord. Data were mixed and not concordant, and studies were retrospective providing class III evidence.
Strahle et al2 performed a retrospective study of 14,118 patients who underwent brain or cervical spine imaging at a single institution over 11 years. Five hundred nine patients had CIM, and CIM was not independently associated with scoliosis.
Milhorat et al3 performed a retrospective review of 2987 patients with CIM and 289 patients with low lying cerebellar tonsils. Four hundred eight patients with CIM and 182 patients with low tonsils had tethered cord syndrome, as defined by the authors.
Leung et al4,5 retrospectively reviewed 64 patients with CIM and 25 control subjects who all underwent cardiac-gated CINE MRI. Patients with CIM were found to have significantly greater cerebellar tonsillar motion, which decreased after posterior fossa decompression (PFD).
Taylor et al5 retrospectively reviewed 68 patients who underwent PFD at a single institution between 2004 and 2016. Twenty-six patients had syrinx at presentation, and syrinx resolution was associated with an increase in subarachnoid space after surgery.
Milhorat et al6 retrospectively reviewed a cohort of 364 patients with symptomatic CIM who underwent brain and spine imaging. Sixty-five percent had syringomyelia, 42% scoliosis, 12% basilar invagination, and 12% a family history of CIM. Clinical symptoms of CIM included headache, pseudotumor-like episodes, Meniere disease symptoms, lower cranial nerve signs, and myelopathy.
Elster and Chen7 retrospectively reviewed 68 patients with CIM to evaluate clinical symptoms that best correlated with radiographic features. Syringomyelia was found in 40% of patients, most commonly between C4 and C6. Patients with tonsil herniation >12 mm were all symptomatic, and 30% of patients with tonsil 5 mm to 10 mm herniation were asymptomatic.
Bollo et al8 performed a retrospective review of patients with CIM who were operated on between 1995 and 2010. Of 206 patients, 101 had complete preoperative imaging. Of these patients, 19 underwent occipito-cervical fusion. Risk factors for fusion included basilar invagination, Chiari 1.5 malformation, and clivoaxial angle (CXA) <125 degrees.
Tubbs et al9 performed a retrospective review of patients with CIM who were operated on between 1989 and 2010. Of 500 patients, the most common symptoms were headache/neck pain (40%) and scoliosis (18%). Twenty-four percent had retroverted odontoid, 3% Klippel–Feil, and 8% atlas assimilation into the occiput. Three percent had a family member with CIM, 9.6% hydrocephalus, and 57% had syringomyelia. Complications were present in 2.4% of cases.
Kennedy et al10 retrospectively reviewed patients <21 years of age between 1998 and 2013 who underwent total spine MRI after diagnosis of CIM. Of 266 patients, 50% had syrinx and 4.5% had isolated thoracic syrinx.
Tubbs et al11 retrospectively reviewed spine MRI in 26 children between 5 and 16 years of age with CIM. No relationship between conus level and amount of tonsil ectopia was found. Of patients with Conus located at L2-L3 disc or below, all had syrinx.
Sadler et al12 retrospectively reviewed 612 pediatric patients with CIM diagnosed between 2008 and 2018. Seventy percent had “standard, nonsyndromic” CIM. Six percent had a genetic abnormality, including NF1, Sturge–Weber, or Ehlers–Danlos syndrome. Syrinx was found in 40% of patients with hypermobile joints, 40% of patients with ventriculomegaly, and 29% of patients with hydrocephalus. Of the syndromic patients, 4% had multiple congenital anomalies, 8% had skeletal dysplasia, and 17% had central nervous system abnormalities.
Menezes13 retrospectively analyzed 100 patients 3 to 66 years of age with Chiari and primary craniovertebral junction abnormalities. Sixty-six patients with irreducible pathology underwent ventral or ventrolateral decompression with dorsal stabilization. Thirty-four patients had reducible pathology and were treated with dorsal stabilization alone. Eight patients had proatlas remnants and 92 had atlas assimilation. Forty-six patients had syringomyelia and 66 had vertebral segmental defects. Cine flow MRI was helpful to evaluate successful treatment.
Strahle et al14 retrospectively reviewed 825 patients with CIM and syrinx, 30% of whom had scoliosis. Sixteen percent underwent PFD. Nine patients had stable curves, 16 had progression, and 16 had improvement. Younger age at surgery was associated with curve improvement.
McGirt et al15 retrospectively reviewed Cine phase contrast MRI in 130 patients receiving PFD for CIM between 1997 and 2003. Normal preoperative CSF flow was a risk factor for surgical decompression treatment failure. The study found that normal Cine MRI flow may predict patients who do not respond to surgery.
Caldarelli et al16 retrospectively reviewed 30 patients who underwent extradural decompression for CIM. Patients were 2 months to 16 years of age. Syringomyelia was found in 40% of patients. Preoperative symptoms included headache or neck pain, vertigo, weakness, and ataxia.
Krieger et al17 retrospectively reviewed 79 patients over a 10-year period with CIM found during scoliosis evaluations. All patients had syringomyelia. All underwent Chiari decompression. On 6-month postoperative MRI, 89% of patients had significant reduction in syrinx, 6 patients had reoperation for persistent large syrinx, and 2 patients required shunt for hydrocephalus. Seventy percent of patients with CIM and scoliosis with a curve >20 degrees required bracing or spine fusion in addition to CIM decompression.
Brockmeyer et al18 retrospectively reviewed 85 patients who underwent PFD for CIM between 1990 and 2000. Twenty-two patients had CIM, scoliosis, and syringomyelia. Sixty-two percent had curve stabilization or improvement after surgery. Ninety-one percent of patients who were <10 years of age had stabilization of their scoliosis.
Bhangoo and Sgouros19 retrospectively reviewed 36 patients with symptomatic CIM who underwent decompression between 1998 and 2003. Thirteen had scoliosis. Decompression may have prevented curve progression for patients <10 years of age and a Cobb angle <30 degrees.
Muhonen et al20 retrospectively reviewed a prospective database in which 11 patients under 16 years of age had CIM and scoliosis. Eight patients had syringomyelia. PFD and duraplasty (PFDD) was performed. Scoliosis improved in 8 patients, stabilized in 1, progressed in 2, and 1 child needed posterior spinal fusion.
O’Neill et al21 retrospectively reviewed 32 patients between 1997 and 2015 who had scoliosis and CIM. For nonoperated patients who had no other clinical symptoms, scoliosis did not progress.
Mauer et al22 retrospectively reviewed 90 patients with CIM who underwent preoperative Cine MRI. Fifty-nine patients had syrinx. Cine MRI was used on 22 patients. These patients had significant pulsations on Cine MRI.
Fan et al23 retrospectively reviewed 126 patients with CIM, 48 underwent subdural decompression, and 78 had decompression with subarachnoid manipulation. CSF flow dynamics were determined for each patient type.
Lee et al24 retrospectively reviewed 56 patients with CIM who received surgery (mean age 7.9 years). Eight had hydrocephalus, 11 had no syrinx, and 37 had syrinx. Minimal or active intradural manipulation was performed. Extent of intradural procedure did not affect the outcome. Syrinx improved in 86% of cases with syrinx. Scoliosis improved or stabilized in 57% of patients.
Villa et al25 retrospectively reviewed 25 patients, mean age 39 years, between 2012 and 2016, who underwent PFD for CIM. Syrinx was present in 48% of patients. Suboccipital craniotomy with tonsil coagulation and duraplasty was performed. Symptoms resolved in 52%, improved in 20%, and were unchanged in 4%. Syrinx improved in 7 of 12 patients with syrinx.
Menezes et al26 retrospectively reviewed 326 surgically treated patients with CIM. Syringobulbia was identified in 13 patients (4%). Vagus and glossopharyngeal nerve dysfunction was most commonly seen, in addition to more rare dysfunction of trigeminal, abducens, and hypoglossal cranial nerves. Central sleep apnea was seen in 6 patients. An arachnoid veil was seen in 9 patients. Syringobulbia improved in all 13 patients after surgery.
Gad et al27 retrospectively reviewed 108 patients with CIM. Thirty-six percent of patients had syrinx. Some patients had skull base anomalies that the authors attributed to syrinx formation.
Lara-Reyna et al28 retrospectively reviewed 48 patients with CIM and syrinx. The authors graded syrinx into 4 categories. Eighty-nine percent of patients had syrinx improvement.
Strahle et al29 retrospectively reviewed 14,118 patients undergoing brain or cervical spine imaging. Two hundred seventy-one patients with syrinx were identified. CIM was found in 117 patients, and 83 patients had an idiopathic syrinx.
Xie et al30 retrospectively reviewed 87 patients 5 to 18 years of age who had PFD for CIM between 2006 and 2012. Neurologic deficits were found in 51 of 87 patients before surgery, and 72% of patients had improved deficits after surgery. Syrinx resolved in 90% cases after surgery.
Question 1-2. In patients with CIM, are advanced imaging modalities such as Cine MRI helpful to predict benefit from surgical decompression?
Recommendation: In patients with CIM, advanced imaging modalities such as Cine MRI may or may not predict benefit from surgical decompression.
Strength of recommendation: Grade C
Class III Evidence
There were 9 articles (class III studies) evaluating the relationship between the use of advanced imaging of CIM and the prediction of benefit from surgical decompression. In general, results were mixed and not uniform across studies. Data were retrospective and classified as class III evidence.
Sadique et al31 performed a prospective study looking at 39 patients who underwent MRIs before and after surgery measuring peak CSF velocity at the foramen magnum over 2 years. After foramen magnum decompression the peak flow velocities improved, however there was no correlation with improvement in clinical symptoms. In addition, the surgeries were all extradural decompression.
Bapuraj et al32 performed a prospective study to assess the CSF bidirectional motion in CIM in 10 patients before and after decompression surgery, and in 10 control subjects. The amplitude of mean velocity and amplitude of peak velocity were high in patients with CIM. After surgery there was no statistical difference between the postsurgery and volunteer groups, indicating “normalization” of flow amplitude postoperatively along with improvement in symptoms.
McGirt et al33 prospectively studied 33 patients with CIM with headache alone along with CSF flow dynamics. Seventeen of these patients underwent decompression surgery. Occipital headaches associated with flow obstruction on Cine MRI was correlated with better outcomes.
McGirt et al34 performed a retrospective study on 44 consecutive patients undergoing preoperative and postoperative Cine phase-contrast MRI assessing ventral or dorsal CSF flow dynamics. Combined ventral and dorsal CSF flow abnormality on preoperative MRI was significantly associated with a 2.6-fold reduction in the risk of postoperative symptom recurrence (risk ratio 22.6 [95% confidence interval 1.16-4.79], p = .03). Decreased CSF flow ventrally as well as dorsal to the cervico-medullary junction was associated with improved response to PFD.
McGirt et al15 retrospectively reviewed 130 patients with CIM to examine whether CSF flow dynamics assessed by pre- and postoperative Cine phase-contrast MRI could independently predict response to PFD for CIM. Abnormal hindbrain flow was observed in 81% of patients. Patient outcomes were recorded at approximately 1 month, 1 year, and at most recent follow-up after surgery. Postoperatively, Cine flow improved in 95 (91%) patients with abnormal CSF flow preoperatively. One month after surgery, 89% of patients demonstrated improvement in symptoms, which decreased to 71% and 67% at 1 and 2 years of follow-up, respectively.
Ventureyra et al35 performed a retrospective study reviewing 22 patients between 2009 and 2013. MRI was conducted to assess tonsillar pulsatility and correlate it with the clinical outcomes after PFD. Eighteen patients underwent PFDD and 4 patients underwent bony PFD. Postoperative MRIs were done at a mean time interval of 17 weeks. The Chicago Chiari Outcome Scale was used for the symptom assessment and ranged between 9 and 16 for all patients and did not show a statistically significant correlation with the amount of change in tonsillar pulsatility after surgery (P values .53, .32, and .10 for 3 readers).
Lara-Reyna et al28 retrospectively reviewed 24 patients to analyze the role of Cine flow MRI in CIM between 1990 and 2000. Sixteen of 24 patients underwent 18 PFD procedures. Symptomatic patients with abnormal MRI Cine flow studies showed both clinical and imaging improvements after surgical intervention; on the other hand, asymptomatic patients with normal MRI Cine flow studies did well without surgical intervention.
Radmanesh et al36 performed a retrospective review of 48 patients with CIM and syringomyelia to introduce a grading system focusing on syrinx reduction based on routinely and reproducible radiologic information, providing a suggestion of the application of this scale for prediction patient’s prognosis. The percentage change was grouped into 4 grades: grade 0: increasing size; grade I: ≤50% reduction; grade II: 50% to 90% reduction; and grade II: ≥90% reduction. Most (89.6%) patients had syrinx improvement after surgery. Five patients were grade 0, 14 were grade I, 20 were grade II, and 9 were grade III.
Ellenbogen et al37 prospectively tested the validity of using cardiac-gated phase-contrast Cine-mode MRI to define the malformation, delineate its pathophysiology, and assist in implementing a rational treatment plan between 1990 and 1999. Sixty-five of 85 patients with CIM with or without syrinx underwent surgical intervention. Twenty healthy individuals were also studied. Compared with control subjects, CIM patients with/without syringomyelia uniformly had craniocervical junction CSF flow abnormalities, and after PFDD, nearly all experienced clinical improvement (pediatric: 64% good, 33% improved, and 3% poor; adults: 69% good, 28% improved, and 3% poor) as well as CSF flow profiles paralleling those of the normal volunteers.
Question 1-3. In patients with CIM, should flexion and extension radiographs be routinely performed to evaluate for cervical instability? Should preoperative evaluation include any specific evaluation for cranial cervical instability or ventral compression (clivoaxial angle, pB-C2, etc)?
Recommendation: In patients with CIM, measurement of the clivoaxial angle, pB-C2, and C-C2SVA may predict future craniocervical instability and the need for surgical stabilization.
Strength of recommendation: Grade C
Class III Evidence
There is insufficient evidence to support the use of flexion-extension films to predict future craniocervical instability in this population.
Strength of recommendation: Grade insufficient
Insufficient Evidence
There were 4 articles (class III studies) evaluating the relationship between various craniocervical metrics and the need for surgical stabilization in patients with CIM. No article directly evaluated the use of flexion-extension films to predict future craniocervical instability in this population. In general, patients requiring surgical stabilization were more likely to have Chiari 1.5, pB-C2 ≥9 mm, CXA <125 mm, Klippel–Feil, and basilar invagination. In many recent articles, the association of these conditions with CIM are called “complex Chiari I malformation.”
Bollo et al8 retrospectively reviewed 101 patients with CIM and 1.5 who underwent PFD alone or PFD and fusion (OCF) either upfront or in a delayed fashion. Eighty-two patients underwent PFD alone, while 19 underwent OCF; of these 19, 11 had upfront OCF and 8 were performed in a delayed fashion at a mean of 4.1 years after PFD (range 1.3-9.2 years). Across all patients, those undergoing OCF had significantly higher proportion of CM 1.5, medullary kink, retroflexed odontoid, basilar invagination, and presence of pB-C2 >9 mm (posterior basion to inferior cervical 2) was significantly higher, presence of CXA <125 degrees was significantly higher, and there was a greater degree of tonsillar descent. There was a higher proportion of those with pB-C2 ≥9 mm. In the subset of patients undergoing delayed fusion, CM 1.5, basilar invagination, CXA <125 degrees, and higher mean pB-C2 were all predictive of the need for fusion.
Ravindra et al38 retrospectively reviewed 60 patients with CIM to examine a novel measured parameter that may help to predict the risk of instability and need for fusion. Seven patients underwent odontoid resection or OCF, and 10 patients required >2 decompressive procedures. They examined the occipital condyle–C2SVA and found that the sensitivity and specificity for requiring ventral decompression (VD) or OCF was 100% and 74%, respectively. Sensitivity and specificity for CXA <125 degrees was 71% and 94%, and sensitivity and specificity for pB-C2 ≥9 mm was 71% and 75%. Notably, these patients all underwent fixation at the time of their index PFD.
CreveCoeur et al39 reviewed 637 patients as part of the Park–Reeves syringomyelia consortium who underwent PFD for CIM. Of these, 12 underwent OCF (9 upfront, 3 delayed), and 4 patients underwent VD and OCF (2 upfront, 2 delayed). Across all patients, platybasia, Klippel–Feil, and basilar invagination were all significantly higher in the OCF group. Basilar invagination was also significantly more common in the OCF/VD group. CXA was significantly lower in the OCF only (125.8 ± 15.3) and OCF/VD group (115.0 ± 11.6) compared with PFD only (145.3 ± 12.9). There is no significant difference in the CXA between those undergoing upfront versus delayed fusion; however, pB-C2 was significantly less at presentation in those undergoing delayed fusion, although this only represents 3 patients in the OCF group.
Grabb et al40 reviewed 40 patients with CIM to examine the relationship of ventral brainstem compression (VBSC) and the role of pB-C2 in determining the need for additional procedures. There was flattening of the brainstem and distortion of the brainstem in 48% and 28%, respectively. Those with pB-C2 <9 mm underwent PFD alone, and those >9 mm had symptoms from VBSC after PFD. Four patients required traction before any procedure, and 3 patients underwent OCF, while 1 patient had odontoidectomy and OCF. One of 3 patients undergoing OCF required further odontoidectomy because of ongoing symptoms. Conversely, 7 patients with pB-C2 >9 mm underwent PFD alone with no need for further reduction or fusion of VBSC. No patients with pB-C2 <9 mm required any additional procedures other than PFD.
Future Research
This review demonstrates numerous gaps in our knowledge about the diagnosis of CIM. To remedy this deficiency, we need well-designed prospective data regarding the preoperative imaging work-up, including the use of advanced imaging to help diagnose patients who may benefit from surgery. Prospective studies are also needed on cranocervical junction metrics and dynamic imaging to predict benefit from, or future need for, craniocervical fusion.
Future studies and collaborative efforts may offer more insights to improve our management approach. Patient-centered studies and the evaluation of patient-reported outcomes may be helpful to inform future clinical decision making and recommendations. It is imperative to explore these questions to help improve care of patients with CIM and syringomyelia.
CONCLUSIONS
There is a significant need for additional high-quality evidence of the diagnostic work-up of CIM. Current low-quality studies provide some evidence that imaging of the entire neuraxis may be helpful to diagnose associated pathology, advanced imaging may or may not be helpful, and current craniocervical metrics may be helpful to predict future need for craniocervical fusion.
Conflicts of Interest
All Guideline Task Force members were required to disclose all potential COIs prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Review Committee. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination and participation on the task force. The CNS Guidelines Committee and Guideline Task Force Chair may approve nominations of task force members with possible conflicts and restrict the writing, reviewing, and/or voting privileges of that person to topics that are unrelated to the possible COIs. See Appendix V for a complete list of disclosures.
Disclosure of Funding
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document.
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a physician volunteer task force as an educational tool that reflects the current state of knowledge at the time of completion. Each chapter is designed to provide an accurate review of the subject matter covered. This guideline is disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient’s physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Acknowledgments
The guidelines task force would like to acknowledge the CNS Guidelines Committee for their contributions throughout the development of the guideline, the AANS/CNS Joint Guidelines Review Committee, as well as the contributions of Kirsten Aquino, contracted project manager for the CNS, Trish Rehring, MPH, Associate Director for Evidence-Based Practice Initiatives for the CNS, and Janet Waters, MLS, BSN, RN, for assistance with the literature searches. The guidelines task force would also like to acknowledge the contributions of Dorothy Poppe, Kaitlyn Esposito, MPH and Mary Poppe, as well as the Bobby Jones Chiari & Syringomyelia Foundation for serving as patient advocates on this guideline task force. Throughout the review process, the reviewers and authors were blinded from one another. At this time the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Jennifer Sweet, MD, Andrew Carlson, MD, MS, Matthew Reynolds, MD, PhD, Alexandra D. Beier, D.O., FACOS, FAAP, Jonathan Pindrik, MD and Patti Raksin, MD.
REFERENCES
1. Ransohoff DF, M. Pignone, and H.C. Sox, . How to decide whether a clinical practice guideline is trustworthy. JAMA. 2013;309(2):139-140.
2. Strahle J, Smith BW, Martinez M, et al. The association between Chiari malformation type I, spinal syrinx, and scoliosis. Journal of neurosurgery Pediatrics. 2015;15(6):607-611.
3. Milhorat TH, Bolognese PA, Nishikawa M, et al. Association of Chiari malformation type I and tethered cord syndrome: preliminary results of sectioning filum terminale. Surgical neurology. 2009;72(1):20-35.
4. Leung V, Magnussen JS, Stoodley MA, Bilston LE. Cerebellar and hindbrain motion in Chiari malformation with and without syringomyelia. Journal of neurosurgery Spine. 2016;24(4):546-555.
5. Taylor DG, Chatrath A, Mastorakos P, et al. Cerebrospinal fluid area and syringogenesis in Chiari malformation type I. Journal of neurosurgery. 2020:1-6.
6. Milhorat TH, Chou MW, Trinidad EM, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44(5):1005-1017.
7. Elster AD, Chen MY. Chiari I malformations: clinical and radiologic reappraisal. Radiology. 1992;183(2):347-353.
8. Bollo RJ, Riva-Cambrin J, Brockmeyer MM, Brockmeyer DL. Complex Chiari malformations in children: an analysis of preoperative risk factors for occipitocervical fusion. Journal of neurosurgery Pediatrics. 2012;10(2):134-141.
9. Tubbs RS, Beckman J, Naftel RP, et al. Institutional experience with 500 cases of surgically treated pediatric Chiari malformation Type I. Journal of neurosurgery Pediatrics. 2011;7(3):248-256.
10. Kennedy BC, Kelly KM, Anderson RC, Feldstein NA. Isolated thoracic syrinx in children with Chiari I malformation. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2016;32(3):531-534.
11. Tubbs RS, Elton S, Bartolucci AA, Grabb P, Oakes WJ. The position of the conus medullaris in children with a Chiari I malformation. Pediatric neurosurgery. 2001;33(5):249-251.
12. Sadler B, Kuensting T, Strahle J, et al. Prevalence and impact of underlying diagnosis and comorbidities on Chiari 1 malformation. Pediatric neurology. 2020;106:32-37.
13. Menezes AH. Primary craniovertebral anomalies and the hindbrain herniation syndrome (Chiari I): data base analysis. Pediatric neurosurgery. 1995;23(5):260-269.
14. Strahle JM, Taiwo R, Averill C, et al. Radiological and clinical associations with scoliosis outcomes after posterior fossa decompression in patients with Chiari malformation and syrinx from the Park-Reeves Syringomyelia Research Consortium. Journal of neurosurgery Pediatrics. 2020:1-7.
15. McGirt MJ, Nimjee SM, Fuchs HE, George TM. Relationship of cine phase-contrast magnetic resonance imaging with outcome after decompression for Chiari I malformations. Neurosurgery. 2006;59(1):140-146; discussion 140-146.
16. Caldarelli M, Novegno F, Vassimi L, Romani R, Tamburrini G, Di Rocco C. The role of limited posterior fossa craniectomy in the surgical treatment of Chiari malformation type I: experience with a pediatric series. Journal of neurosurgery. 2007;106(3 Suppl):187-195.
17. Krieger MD, Falkinstein Y, Bowen IE, Tolo VT, McComb JG. Scoliosis and Chiari malformation type I in children. Journal of neurosurgery Pediatrics. 2011;7(1):25-29.
18. Brockmeyer D, Gollogly S, Smith JT. Scoliosis associated with Chiari 1 malformations: the effect of suboccipital decompression on scoliosis curve progression: a preliminary study. Spine. 2003;28(22):2505-2509.
19. Bhangoo R, Sgouros S. Scoliosis in children with Chiari I-related syringomyelia. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2006;22(9):1154-1157.
20. Muhonen MG, Menezes AH, Sawin PD, Weinstein SL. Scoliosis in pediatric Chiari malformations without myelodysplasia. Journal of neurosurgery. 1992;77(1):69-77.
21. O'Neill NP, Miller PE, Hresko MT, et al. Scoliosis with Chiari I malformation without associated syringomyelia. Spine deformity. 2021.
22. Mauer UM, Gottschalk A, Mueller C, Weselek L, Kunz U, Schulz C. Standard and cardiac-gated phase-contrast magnetic resonance imaging in the clinical course of patients with Chiari malformation Type I. Neurosurgical focus. 2011;31(3):E5.
23. Fan T, Zhao H, Zhao X, Liang C, Wang Y, Gai Q. Surgical management of Chiari I malformation based on different cerebrospinal fluid flow patterns at the cranial-vertebral junction. Neurosurgical review. 2017;40(4):663-670.
24. Lee S, Wang KC, Cheon JE, et al. Surgical outcome of Chiari I malformation in children: clinico-radiological factors and technical aspects. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2014;30(4):613-623.
25. Villa A, Imperato A, Maugeri R, Visocchi M, Iacopino DG, Francaviglia N. Surgical treatment in symptomatic Chiari malformation type I: a series of 25 adult patients treated with cerebellar tonsil shrinkage. Acta neurochirurgica Supplement. 2019;125:125-131.
26. Menezes AH, Greenlee JDW, Dlouhy BJ. Syringobulbia in pediatric patients with Chiari malformation type I. Journal of neurosurgery Pediatrics. 2018;22(1):52-60.
27. Gad KA, Yousem DM. Syringohydromyelia in patients with Chiari I malformation: a retrospective analysis. AJNR American journal of neuroradiology. 2017;38(9):1833-1838.
28. Lara-Reyna J, Chae J, Tosi U, Souweidane MM, Uribe-Cardenas R, Greenfield JP. Syringomyelia resolution following Chiari surgery: a novel scale for communication and research. Neurosurgery. 2020;88(1):E60-e66.
29. Strahle J, Muraszko KM, Garton HJ, et al. Syrinx location and size according to etiology: identification of Chiari-associated syrinx. Journal of neurosurgery Pediatrics. 2015;16(1):21-29.
30. Xie D, Qiu Y, Sha S, et al. Syrinx resolution is correlated with the upward shifting of cerebellar tonsil following posterior fossa decompression in pediatric patients with Chiari malformation type I. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2014;24(1):155-161.
31. Sadique SI, Pandey P, Chaudhuri AK. Cerebrospinal fluid flowmetry in pediatric patients with Chiari malformation I with surgical implications. World Neurosurgery. 2019;135:e83-e86.
32. Bapuraj JR, Londy FJ, Delavari N, et al. Cerebrospinal fluid velocity amplitudes within the cerebral aqueduct in healthy children and patients with Chiari I malformation. Journal of magnetic resonance imaging : JMRI. 2016;44(2):463-470.
33. McGirt MJ, Nimjee SM, Floyd J, Bulsara KR, George TM. Correlation of cerebrospinal fluid flow dynamics and headache in Chiari I malformation. Neurosurgery. 2005;56(4):716-721; discussion 716-721.
34. McGirt MJ, Atiba A, Attenello FJ, et al. Correlation of hindbrain CSF flow and outcome after surgical decompression for Chiari I malformation. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2008;24(7):833-840.
35. Ventureyra EC, Aziz HA, Vassilyadi M. The role of cine flow MRI in children with Chiari I malformation. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2003;19(2):109-113.
36. Radmanesh A, Greenberg JK, Chatterjee A, Smyth MD, Limbrick DD, Jr., Sharma A. Tonsillar pulsatility before and after surgical decompression for children with Chiari malformation type 1: an application for true fast imaging with steady state precession. Neuroradiology. 2015;57(4):387-393.
37. Ellenbogen RG, Armonda RA, Shaw DW, Winn HR. Toward a rational treatment of Chiari I malformation and syringomyelia. Neurosurgical focus. 2006;8(3):E6.
38. Ravindra VM, Iyer RR, Awad AW, Bollo RJ, Zhu H, Brockmeyer DL. Defining the role of the condylar-C2 sagittal vertical alignment in Chiari malformation type I. Journal of neurosurgery Pediatrics. 2020:1-6.
39. CreveCoeur TS, Yahanda AT, Maher CO, et al. Occipital-cervical fusion and ventral decompression in the surgical management of Chiari-1 malformation and syringomyelia: analysis of data from the Park-Reeves Syringomyelia Research Consortium. Neurosurgery. 2020;88(2):332-341.
40. Grabb PA, Mapstone TB, Oakes WJ. Ventral brain stem compression in pediatric and young adult patients with Chiari I malformations. Neurosurgery. 1999;44(3):520-527; discussion 527-528.
41. Goel A, Gore S, Shah A, Dharurkar P, Vutha R, Patil A. Atlantoaxial fixation for Chiari 1 formation in pediatric age-group patients: report of treatment in 33 patients. World neurosurgery. 2018;111:e668-e677.
Appendix I. Literature searches
Search Strategies
PUBMED SEARCH STRATEGY
(((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE] AND ("THORACIC VERTEBRAE/DIAGNOSTIC IMAGING"[MESH TERMS] OR "THORACIC IMAGING*"[TITLE/ABSTRACT] OR "LUMBAR VERTEBRAE/DIAGNOSTIC IMAGING"[MESH TERMS] OR "LUMBAR IMAGING*"[TITLE/ABSTRACT] OR (("THORACIC VERTEBRAE"[MESH TERMS] OR "THORACIC VERTEBRA*"[TITLE/ABSTRACT] OR "THORACIC SPINE"[TITLE/ABSTRACT] OR "THORACIC VERTEBRAL"[TITLE/ABSTRACT] OR "THORAX SPINE"[TITLE/ABSTRACT] OR "LUMBAR VERTEBRAE"[MESH TERMS] OR "LUMBAR VERTEBRA*"[TITLE/ABSTRACT] OR "LUMBAR SPINE*"[TITLE/ABSTRACT] OR "LUMBAR VERTEBRAL"[TITLE/ABSTRACT] OR "VERTEBRAE LUMBALES"[TITLE/ABSTRACT]) AND ("DIAGNOSTIC IMAGING"[MESH TERMS:NOEXP] OR "IMAGING*"[TEXT WORD] OR "MAGNETIC RESONANCE IMAGING"[MESH TERMS:NOEXP] OR "MAGNETIC RESONANCE*"[TITLE/ABSTRACT] OR "MRI"[TITLE/ABSTRACT] OR "MRIS"[TITLE/ABSTRACT] OR "MR TOMOGRAPH*"[TITLE/ABSTRACT] OR "NMR TOMOGRAPH*"[TITLE/ABSTRACT] OR "ZEUGMATOGRAPH*"[TITLE/ABSTRACT] OR "PROTON SPIN TOMOGRAPH*"[TITLE/ABSTRACT] OR "FMRI"[TITLE/ABSTRACT])) OR ("GLIOMA/DIAGNOSTIC IMAGING"[MESH TERMS] OR "BRAIN NEOPLASMS/DIAGNOSTIC IMAGING"[MESH TERMS] OR (("GLIOMA*"[TITLE/ABSTRACT] OR "GLIAL CELL TUMOR*"[TITLE/ABSTRACT] OR "GLIAL CELL TUMOUR*"[TITLE/ABSTRACT] OR "BRAIN NEOPLASM*"[TITLE/ABSTRACT] OR "BRAIN LESION*"[TITLE/ABSTRACT] OR "BRAIN TUMOR*"[TITLE/ABSTRACT] OR "BRAIN TUMOUR*"[TITLE/ABSTRACT] OR "BRAIN CANCER*"[TITLE/ABSTRACT] OR "INTRACRANIAL NEOPLASM*"[TITLE/ABSTRACT] OR "CEREBELLUM/PATHOLOGY"[MESH TERMS] OR "TONSIL*"[TEXT WORD]) AND ("IMAGING*"[TEXT WORD] OR "MAGNETIC RESONANCE IMAGING"[MESH TERMS:NOEXP] OR "MAGNETIC RESONANCE*"[TEXT WORD] OR "NEUROIMAGING"[MESH TERMS] OR "NEUROIMAG*"[TITLE/ABSTRACT] OR "NEURO IMAG*"[TITLE/ABSTRACT] OR "MRI"[TITLE/ABSTRACT] OR "MRIS"[TITLE/ABSTRACT] OR "MR TOMOGRAPH*"[TITLE/ABSTRACT] OR "NMR TOMOGRAPH*"[TITLE/ABSTRACT] OR "ZEUGMATOGRAPH*"[TITLE/ABSTRACT] OR "PROTON SPIN TOMOGRAPH*"[TITLE/ABSTRACT] OR "FMRI"[TITLE/ABSTRACT])) OR ((("HYDROCEPHAL*"[TITLE/ABSTRACT] OR "CEREBRAL VENTRICULOMEGAL*"[TITLE/ABSTRACT] OR "AQUEDUCTAL STENOS*"[TITLE/ABSTRACT]) AND ("IMAGING*"[TEXT WORD] OR "MAGNETIC RESONANCE IMAGING"[MESH TERMS:NOEXP] OR "MAGNETIC RESONANCE*"[TITLE/ABSTRACT] OR "NEUROIMAGING"[MESH TERMS] OR "NEUROIMAG*"[TITLE/ABSTRACT] OR "NEURO IMAG*"[TITLE/ABSTRACT] OR "MRI"[TITLE/ABSTRACT] OR "MRIS"[TITLE/ABSTRACT] OR "MR TOMOGRAPH*"[TITLE/ABSTRACT] OR "NMR TOMOGRAPH*"[TITLE/ABSTRACT] OR "ZEUGMATOGRAPH*"[TITLE/ABSTRACT] OR "PROTON SPIN TOMOGRAPH*"[TITLE/ABSTRACT] OR "FMRI"[TITLE/ABSTRACT])) OR "HYDROCEPHALUS/DIAGNOSTIC IMAGING"[MESH TERMS]) OR ("SYRINGOMYELIA/DIAGNOSTIC IMAGING"[MESH TERMS] OR (("SYRINGOMYELIA*"[TITLE/ABSTRACT] OR "SYRINGOMYELUS*"[TITLE/ABSTRACT] OR "MYELOSYRINGOS*"[TITLE/ABSTRACT] OR "MORVAN DISEASE"[TITLE/ABSTRACT] OR "MORVAN S DISEASE*"[TITLE/ABSTRACT] OR "HYDROSYRINGOMYELIA*"[TITLE/ABSTRACT] OR "SYRINX*"[TITLE/ABSTRACT]) AND ((("IMAGING*"[TEXT WORD] OR "MAGNETIC RESONANCE IMAGING"[MESH TERMS:NOEXP] OR "MAGNETIC RESONANCE*"[TITLE/ABSTRACT]) AND "MRI"[TITLE/ABSTRACT]) OR "MRIS"[TITLE/ABSTRACT] OR "MR TOMOGRAPH*"[TITLE/ABSTRACT] OR "NMR TOMOGRAPH*"[TITLE/ABSTRACT] OR "ZEUGMATOGRAPH*"[TITLE/ABSTRACT] OR "PROTON SPIN TOMOGRAPH*"[TITLE/ABSTRACT] OR "FMRI"[TITLE/ABSTRACT]))) OR (("IMAGING*"[TEXT WORD] OR "MAGNETIC RESONANCE IMAGING"[MESH TERMS:NOEXP] OR "MAGNETIC RESONANCE*"[TITLE/ABSTRACT] OR "MRI"[TITLE/ABSTRACT] OR "MRIS"[TITLE/ABSTRACT] OR "MR TOMOGRAPH*"[TITLE/ABSTRACT] OR "NMR TOMOGRAPH*"[TITLE/ABSTRACT] OR "ZEUGMATOGRAPH*"[TITLE/ABSTRACT] OR "PROTON SPIN TOMOGRAPH*"[TITLE/ABSTRACT] OR "FMRI"[TITLE/ABSTRACT]) AND ("NEURAL TUBE DEFECTS"[MESH TERMS:NOEXP] OR "TETHERED CORD*"[TITLE/ABSTRACT] OR ("TETHERED SPINAL CORD*"[TITLE/ABSTRACT] OR "TETHERING CORD*"[TITLE/ABSTRACT])))))) OR (((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE] AND ((("RANGE OF MOTION"[TITLE/ABSTRACT] OR "FLEXION*"[TITLE/ABSTRACT] OR "EXTENSION*"[TITLE/ABSTRACT] OR "RANGE OF MOTION, ARTICULAR"[MESH TERMS] OR "JOINT FLEXIBILITY"[TITLE/ABSTRACT]) AND ("IMAGING*"[TEXT WORD] OR "MAGNETIC RESONANCE IMAGING"[MESH TERMS:NOEXP] OR "MRI"[TITLE/ABSTRACT] OR "MRIS"[TITLE/ABSTRACT] OR "MR TOMOGRAPH*"[TITLE/ABSTRACT] OR "NMR TOMOGRAPH*"[TITLE/ABSTRACT] OR "ZEUGMATOGRAPH*"[TITLE/ABSTRACT] OR "PROTON SPIN TOMOGRAPH*"[TITLE/ABSTRACT] OR "FMRI"[TITLE/ABSTRACT])) OR ("CINE FLOW*"[TITLE/ABSTRACT] OR "4 D FLOW"[TITLE/ABSTRACT] OR "MAGNETIC RESONANCE IMAGING, CINE"[MESH TERMS] OR "CINE MRI"[TITLE/ABSTRACT] OR "CINE MRIS"[TITLE/ABSTRACT] OR "CINE MAGNETIC RESONANCE IMAGING*"[TITLE/ABSTRACT]))) OR (("RADIOGRAPHY"[MESH TERMS:NOEXP] OR "RADIOLOG*"[TEXT WORD] OR "RADIOGRAPH*"[TEXT WORD] OR "NEURORADIOGRAPHY"[MESH TERMS:NOEXP] OR "NEURORADIOGRAPH*"[TITLE/ABSTRACT] OR "NEURO RADIOGRAPH*"[TITLE/ABSTRACT] OR "NEURORADIOLOG*"[TITLE/ABSTRACT] OR "NEURO RADIOLOG*"[TITLE/ABSTRACT] OR "X RAY*"[TITLE/ABSTRACT] OR "XRAY*"[TITLE/ABSTRACT] OR "ROENTGENOGRAPH*"[TITLE/ABSTRACT] OR "IMAGING*"[TEXT WORD]) AND ("FLEXION EXTENSION*"[TITLE/ABSTRACT] OR "HYPEREXTENSION*"[TITLE/ABSTRACT] OR "FLEXION AND EXTENSION"[TITLE/ABSTRACT] OR "RANGE OF MOTION"[TITLE/ABSTRACT] OR "RANGE OF MOTION, ARTICULAR"[MESH TERMS]) AND ((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE]) OR (((("RADIOGRAPHY"[MESH TERMS:NOEXP] OR "RADIOLOG*"[TEXT WORD] OR "RADIOGRAPH*"[TEXT WORD] OR "NEURORADIOGRAPHY"[MESH TERMS:NOEXP] OR "NEURORADIOGRAPH*"[TITLE/ABSTRACT] OR "NEURO RADIOGRAPH*"[TITLE/ABSTRACT] OR "NEURORADIOLOG*"[TITLE/ABSTRACT] OR "NEURO RADIOLOG*"[TITLE/ABSTRACT] OR "X RAY*"[TITLE/ABSTRACT] OR "XRAY*"[TITLE/ABSTRACT] OR "ROENTGENOGRAPH*"[TITLE/ABSTRACT] OR "IMAGING*"[TEXT WORD]) AND ("FLEXION EXTENSION*"[TITLE/ABSTRACT] OR "HYPEREXTENSION*"[TITLE/ABSTRACT] OR "FLEXION AND EXTENSION"[TITLE/ABSTRACT] OR "RANGE OF MOTION"[TITLE/ABSTRACT] OR "RANGE OF MOTION, ARTICULAR"[MESH TERMS]) AND ((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE]) OR ((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE]))) AND "ENGLISH"[LANGUAGE] AND ("BASILAR IMPRESSION PRIMARY"[SUPPLEMENTARY CONCEPT] OR "BASILAR IMPRESSION*"[TITLE/ABSTRACT] OR "BASILAR INVAGINATION*"[TITLE/ABSTRACT] OR "EHLERS DANLOS SYNDROME"[MESH TERMS] OR "EHLERS DANLOS"[TITLE/ABSTRACT] OR "CUTIS ELASTICA"[TITLE/ABSTRACT] OR "EDS IV"[TITLE/ABSTRACT] OR "PLATYBASIA"[MESH TERMS] OR "PLATYBASIA*"[TITLE/ABSTRACT] OR "NECK INSTABILITY"[TITLE/ABSTRACT] OR "CERVICAL INSTABILITY"[TITLE/ABSTRACT] OR "CVJ INSTABILIT*"[TITLE/ABSTRACT] OR "CRANIO VERTEBRAL INSTABILITY"[TITLE/ABSTRACT] OR "CRANIOVERTEBRAL INSTABILITY"[TITLE/ABSTRACT] OR "CRANIO VERTEBRAL JUNCTION INSTABILITY"[TITLE/ABSTRACT] OR "CRANIOVERTEBRAL JUNCTION INSTABILITY"[TITLE/ABSTRACT] OR "CRANIOCERVICAL INSTABILIT*"[TITLE/ABSTRACT] OR "CRANIO CERVICAL INSTABILIT*"[TITLE/ABSTRACT] OR "CRANIAL CERVICAL INSTABILIT*"[TITLE/ABSTRACT] OR "OBEX"[TITLE/ABSTRACT] OR "CRANIO CERVICAL JUNCTION*"[TITLE/ABSTRACT] OR "CRANIOCERVICAL JUNCTION*"[TITLE/ABSTRACT] OR (("CERVICAL VERTEBRAE"[MESH TERMS] OR "CERVICAL VERTEBRA*"[TITLE/ABSTRACT] OR "CERVICAL SPINE"[TITLE/ABSTRACT] OR "CERVICAL ATLAS"[TITLE/ABSTRACT] OR "C1 VERTEBRA*"[TITLE/ABSTRACT] OR "ARCUATE FORAMEN"[TITLE/ABSTRACT] OR "PONTICULUS POSTICUS"[TITLE/ABSTRACT] OR "KIMMERLE ANOMALY"[TITLE/ABSTRACT] OR "PONTICULUS POSTERIOR OF THE ATLAS"[TITLE/ABSTRACT] OR "ODONTOID PROCESS*"[TITLE/ABSTRACT] OR "DENS AXIS"[TITLE/ABSTRACT] OR "C2 VERTEBRA*"[TITLE/ABSTRACT] OR "EPISTROPHEUS"[TITLE/ABSTRACT] OR "OS ODONTOIDEUM"[TITLE/ABSTRACT] OR "CRANIO CERVICAL"[TITLE/ABSTRACT] OR "CRANIOCERVICAL"[TITLE/ABSTRACT] OR "CRANIAL CERVICAL"[TITLE/ABSTRACT] OR "ATLANTO AXIAL JOINT"[MESH TERMS] OR "ATLANTO AXIAL"[TITLE/ABSTRACT] OR "ATLANTOAXIAL"[TITLE/ABSTRACT] OR "ATLANTO OCCIPITAL JOINT"[MESH TERMS] OR "ATLANTO OCCIPITAL"[TITLE/ABSTRACT] OR "ATLOIDO OCCIPITAL JOINT*"[TITLE/ABSTRACT] OR "OCCIPITOATLANTOAXIAL*"[TITLE/ABSTRACT] OR "OCCIPITOCERVICAL*"[TITLE/ABSTRACT] OR "OCCIPITAL CERVICAL"[TITLE/ABSTRACT]) AND ("INSTABILITY"[TEXT WORD] OR "HYPERMOBIL*"[TITLE/ABSTRACT])))) OR (("VBSC"[TITLE/ABSTRACT] OR "CLIVUS AXIS"[TITLE/ABSTRACT] OR "CLIVAL*"[TITLE/ABSTRACT] OR "PB C2"[TITLE/ABSTRACT] OR "CLIVOAXIAL ANGLE*"[TITLE/ABSTRACT] OR "CLIVO AXIAL ANGLE*"[TITLE/ABSTRACT] OR "CXA"[TITLE/ABSTRACT] OR ((("BRAIN STEM"[MESH TERMS] OR "BRAIN STEM*"[TITLE/ABSTRACT] OR "BRAINSTEM*"[TITLE/ABSTRACT] OR "TRUNCUS CEREBRI"[TITLE/ABSTRACT]) AND "COMPRESSION*"[TEXT WORD]) OR "VENTRAL COMPRESSION*"[TITLE/ABSTRACT])) AND ((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE]) OR (("PFDD"[TITLE/ABSTRACT] OR "PFD"[TITLE/ABSTRACT] OR "PFDRT"[TITLE/ABSTRACT] OR "PFBD"[TITLE/ABSTRACT] OR "PFBDD"[TITLE/ABSTRACT] OR "DURA MATER/SURGERY"[MESH TERMS] OR "DURA SPLITTING*"[TITLE/ABSTRACT] OR "DURA MATER SURGER*"[TITLE/ABSTRACT] OR "DURA MATER TRANSPLANT*"[TITLE/ABSTRACT] OR "DURA MATER/TRANSPLANTATION"[MESH TERMS] OR "DURAPLAST*"[TITLE/ABSTRACT] OR "DECOMPRESSION, SURGICAL"[MESH TERMS:NOEXP] OR "DECOMPRESSIVE CRANIECTOMY"[MESH TERMS] OR "CRANIAL FOSSA, POSTERIOR/SURGERY"[MESH TERMS] OR "BONY DECOMPRESSION*"[TITLE/ABSTRACT] OR "BONE ONLY DECOMPRESSION"[TITLE/ABSTRACT] OR "FORAMEN MAGNUM/SURGERY"[MESH TERMS] OR "FORAMEN MAGNUM DECOMPRESSION"[TITLE/ABSTRACT] OR "FORAMEN MAGNUM SURGER*"[TITLE/ABSTRACT] OR "OCCIPITOCERVICAL FIXATION*"[TITLE/ABSTRACT] OR "ATLANTO OCCIPITAL JOINT/SURGERY"[MESH TERMS] OR "OCCIPITOCERVICAL FUSION*"[TITLE/ABSTRACT] OR (("DECOMPRESSION"[TITLE/ABSTRACT] OR "DECOMPRESSIVE"[TITLE/ABSTRACT]) AND ("FOSSA CRANII POSTERIOR"[TITLE/ABSTRACT] OR "FOSSA POSTERIOR"[TITLE/ABSTRACT] OR "POSTERIOR CEREBRAL FOSSA*"[TITLE/ABSTRACT] OR "CRANIAL FOSSA, POSTERIOR"[MESH TERMS] OR "POSTERIOR CRANIAL FOSSA*"[TITLE/ABSTRACT] OR "POSTERIOR FOSSA*"[TITLE/ABSTRACT] OR "CLIVUS"[TITLE/ABSTRACT] OR "DURAL SUBSTITUTE*"[TITLE/ABSTRACT] OR "AUTOLOGOUS GRAFT*"[TITLE/ABSTRACT] OR "NONAUTOLOGOUS GRAFT*"[TITLE/ABSTRACT] OR "NON AUTOLOGOUS GRAFT*"[TITLE/ABSTRACT] OR "DURAL GRAFT*"[TITLE/ABSTRACT] OR "DURASEAL"[TITLE/ABSTRACT] OR "DUREPAIR"[TITLE/ABSTRACT] OR "ENDURA"[TITLE/ABSTRACT] OR "CADAVERIC PERICARDIUM"[TITLE/ABSTRACT] OR "AUTOGRAFTS"[MESH TERMS] OR "AUTOGRAFT*"[TITLE/ABSTRACT] OR "ALLOGRAFTS"[MESH TERMS] OR "ALLOGRAFT*"[TITLE/ABSTRACT])) OR ("SURGER*"[TEXT WORD] OR "SURGICAL*"[TEXT WORD] OR "NEUROSURG*"[TEXT WORD] OR "NEURO SURG*"[TITLE/ABSTRACT])) AND (((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE]) AND ("IMPROV*"[TEXT WORD] OR "RESOLUTION*"[TITLE/ABSTRACT] OR "RESOLV*"[TITLE/ABSTRACT] OR "TREATMENT OUTCOME"[MESH TERMS:NOEXP] OR "OUTCOME*"[TEXT WORD] OR "EFFICAC*"[TITLE/ABSTRACT] OR "EFFECTIV*"[TITLE/ABSTRACT] OR "TREATMENT FAILURE"[MESH TERMS] OR "POSTOPERATIV*"[TEXT WORD] OR "POST OP*"[TITLE/ABSTRACT])) OR (("ASYMPTOMATIC*"[TITLE/ABSTRACT] OR "BENIGN*"[TITLE/ABSTRACT] OR "PRESYMPTOMATIC*"[TITLE/ABSTRACT] OR "PRE SYMPTOMATIC*"[TITLE/ABSTRACT]) AND ("PFDD"[TITLE/ABSTRACT] OR "PFD"[TITLE/ABSTRACT] OR "PFDRT"[TITLE/ABSTRACT] OR "PFBD"[TITLE/ABSTRACT] OR "PFBDD"[TITLE/ABSTRACT] OR "DURA MATER/SURGERY"[MESH TERMS] OR "DURA MATER TRANSPLANT*"[TITLE/ABSTRACT] OR "DURA MATER/TRANSPLANTATION"[MESH TERMS] OR "DURAL SUBSTITUTE*"[TITLE/ABSTRACT] OR "AUTOLOGOUS GRAFT*"[TITLE/ABSTRACT] OR "NONAUTOLOGOUS GRAFT*"[TITLE/ABSTRACT] OR "DURAL GRAFT*"[TITLE/ABSTRACT] OR "DURASEAL"[TITLE/ABSTRACT] OR "DUREPAIR"[TITLE/ABSTRACT] OR "ENDURA"[TITLE/ABSTRACT] OR "CADAVERIC PERICARDIUM"[TITLE/ABSTRACT] OR "AUTOGRAFTS"[MESH TERMS] OR "AUTOGRAFT*"[TITLE/ABSTRACT] OR "ALLOGRAFTS"[MESH TERMS] OR "ALLOGRAFT*"[TITLE/ABSTRACT] OR "DURA SPLITTING*"[TITLE/ABSTRACT] OR "DURA MATER SURGER*"[TITLE/ABSTRACT] OR "DURAPLAST*"[TITLE/ABSTRACT] OR "DECOMPRESSION, SURGICAL"[MESH TERMS:NOEXP] OR "DECOMPRESSIVE CRANIECTOMY"[MESH TERMS] OR "CRANIAL FOSSA, POSTERIOR/SURGERY"[MESH TERMS] OR "BONY DECOMPRESSION*"[TITLE/ABSTRACT] OR "BONE ONLY DECOMPRESSION"[TITLE/ABSTRACT] OR "FORAMEN MAGNUM/SURGERY"[MESH TERMS] OR "FORAMEN MAGNUM DECOMPRESSION"[TITLE/ABSTRACT] OR "FORAMEN MAGNUM SURGER*"[TITLE/ABSTRACT] OR "OCCIPITOCERVICAL FIXATION*"[TITLE/ABSTRACT] OR "ATLANTO OCCIPITAL JOINT/SURGERY"[MESH TERMS] OR "OCCIPITOCERVICAL FUSION*"[TITLE/ABSTRACT] OR (("DECOMPRESSION"[TITLE/ABSTRACT] OR "DECOMPRESSIVE"[TITLE/ABSTRACT]) AND ("FOSSA CRANII POSTERIOR"[TITLE/ABSTRACT] OR "FOSSA POSTERIOR"[TITLE/ABSTRACT] OR "POSTERIOR CEREBRAL FOSSA*"[TITLE/ABSTRACT] OR "CRANIAL FOSSA, POSTERIOR"[MESH TERMS] OR "POSTERIOR CRANIAL FOSSA*"[TITLE/ABSTRACT] OR "POSTERIOR FOSSA*"[TITLE/ABSTRACT] OR "CLIVUS"[TITLE/ABSTRACT])) OR ("SURGER*"[TEXT WORD] OR "SURGICAL*"[TEXT WORD] OR "NEUROSURG*"[TEXT WORD] OR "NEURO SURG*"[TITLE/ABSTRACT] OR "PROPHYLA*"[TITLE/ABSTRACT])) AND ((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE]) OR (((("ASYMPTOMATIC*"[TITLE/ABSTRACT] OR "BENIGN*"[TITLE/ABSTRACT] OR "PRESYMPTOMATIC*"[TITLE/ABSTRACT] OR "PRE SYMPTOMATIC*"[TITLE/ABSTRACT]) AND ("PFDD"[TITLE/ABSTRACT] OR "PFD"[TITLE/ABSTRACT] OR "PFDRT"[TITLE/ABSTRACT] OR "PFBD"[TITLE/ABSTRACT] OR "PFBDD"[TITLE/ABSTRACT] OR "DURA MATER/SURGERY"[MESH TERMS] OR "DURA MATER TRANSPLANT*"[TITLE/ABSTRACT] OR "DURA MATER/TRANSPLANTATION"[MESH TERMS] OR "DURAL SUBSTITUTE*"[TITLE/ABSTRACT] OR "AUTOLOGOUS GRAFT*"[TITLE/ABSTRACT] OR "NONAUTOLOGOUS GRAFT*"[TITLE/ABSTRACT] OR "DURAL GRAFT*"[TITLE/ABSTRACT] OR "DURASEAL"[TITLE/ABSTRACT] OR "DUREPAIR"[TITLE/ABSTRACT] OR "ENDURA"[TITLE/ABSTRACT] OR "CADAVERIC PERICARDIUM"[TITLE/ABSTRACT] OR "AUTOGRAFTS"[MESH TERMS] OR "AUTOGRAFT*"[TITLE/ABSTRACT] OR "ALLOGRAFTS"[MESH TERMS] OR "ALLOGRAFT*"[TITLE/ABSTRACT] OR "DURA SPLITTING*"[TITLE/ABSTRACT] OR "DURA MATER SURGER*"[TITLE/ABSTRACT] OR "DURAPLAST*"[TITLE/ABSTRACT] OR "DECOMPRESSION, SURGICAL"[MESH TERMS:NOEXP] OR "DECOMPRESSIVE CRANIECTOMY"[MESH TERMS] OR "CRANIAL FOSSA, POSTERIOR/SURGERY"[MESH TERMS] OR "BONY DECOMPRESSION*"[TITLE/ABSTRACT] OR "BONE ONLY DECOMPRESSION"[TITLE/ABSTRACT] OR "FORAMEN MAGNUM/SURGERY"[MESH TERMS] OR "FORAMEN MAGNUM DECOMPRESSION"[TITLE/ABSTRACT] OR "FORAMEN MAGNUM SURGER*"[TITLE/ABSTRACT] OR "OCCIPITOCERVICAL FIXATION*"[TITLE/ABSTRACT] OR "ATLANTO OCCIPITAL JOINT/SURGERY"[MESH TERMS] OR "OCCIPITOCERVICAL FUSION*"[TITLE/ABSTRACT] OR (("DECOMPRESSION"[TITLE/ABSTRACT] OR "DECOMPRESSIVE"[TITLE/ABSTRACT]) AND ("FOSSA CRANII POSTERIOR"[TITLE/ABSTRACT] OR "FOSSA POSTERIOR"[TITLE/ABSTRACT] OR "POSTERIOR CEREBRAL FOSSA*"[TITLE/ABSTRACT] OR "CRANIAL FOSSA, POSTERIOR"[MESH TERMS] OR "POSTERIOR CRANIAL FOSSA*"[TITLE/ABSTRACT] OR "POSTERIOR FOSSA*"[TITLE/ABSTRACT] OR "CLIVUS"[TITLE/ABSTRACT])) OR ("SURGER*"[TEXT WORD] OR "SURGICAL*"[TEXT WORD] OR "NEUROSURG*"[TEXT WORD] OR "NEURO SURG*"[TITLE/ABSTRACT] OR "PROPHYLA*"[TITLE/ABSTRACT])) AND ((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE]) OR ("ASYMPTOMATIC*"[TITLE/ABSTRACT] OR "BENIGN*"[TITLE/ABSTRACT] OR "PRESYMPTOMATIC*"[TITLE/ABSTRACT] OR "PRE SYMPTOMATIC*"[TITLE/ABSTRACT])) AND ((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE] AND ("CLINICAL DECISION MAKING"[MESH TERMS] OR "CONSERVATIVE TREATMENT"[MESH TERMS] OR "CONSERVATIVE TREATMENT*"[TITLE/ABSTRACT] OR "CONSERVATIVE MANAGEMENT*"[TITLE/ABSTRACT] OR "CONSERVATIVE THERAP*"[TITLE/ABSTRACT] OR "WATCHFUL WAITING"[MESH TERMS] OR "WATCHFUL WAITING"[TITLE/ABSTRACT] OR "ACTIVE SURVEILLANCE"[TITLE/ABSTRACT] OR "WAIT AND SEE"[TITLE/ABSTRACT] OR "EXPECTANT MANAGEMENT*"[TITLE/ABSTRACT])) OR (((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE] AND ("ACTIVITY RESTRICT*"[TITLE/ABSTRACT] OR "SPORTS"[MESH TERMS] OR "SPORTS"[TITLE/ABSTRACT] OR "SPORT"[TITLE/ABSTRACT] OR "ATHLET*"[TEXT WORD] OR ("FOOTBALL"[MESH TERMS] OR "FOOTBALL"[TITLE/ABSTRACT] OR "RUGBY*"[TITLE/ABSTRACT]) OR ("SOCCER"[MESH TERMS] OR "SOCCER*"[TITLE/ABSTRACT]) OR ("BOXING"[MESH TERMS] OR "BOXING"[TITLE/ABSTRACT]) OR ("WRESTLING"[MESH TERMS] OR "WRESTLING"[TITLE/ABSTRACT]) OR ("WEIGHT LIFTING"[MESH TERMS] OR "WEIGHT LIFTING"[TITLE/ABSTRACT]) OR ("HOCKEY"[MESH TERMS] OR "HOCKEY"[TITLE/ABSTRACT]) OR "LACROSSE"[TITLE/ABSTRACT] OR "HURLING"[TITLE/ABSTRACT] OR "FUTSAL"[TITLE/ABSTRACT] OR "WATER POLO"[TITLE/ABSTRACT] OR "ROLLER DERBY"[TITLE/ABSTRACT] OR "KABADI"[TITLE/ABSTRACT] OR "KABADDI"[TITLE/ABSTRACT] OR ("BASKETBALL"[MESH TERMS] OR "BASKETBALL"[TITLE/ABSTRACT]) OR "QUIDDITCH"[TITLE/ABSTRACT] OR "SHINTY"[TITLE/ABSTRACT] OR ("MARTIAL ARTS"[MESH TERMS] OR "MARTIAL ARTS"[TITLE/ABSTRACT]) OR ("JUDO"[TITLE/ABSTRACT] OR "KARATE"[TITLE/ABSTRACT] OR "JUJITSU"[TITLE/ABSTRACT] OR "TAE KWON DO"[TITLE/ABSTRACT] OR "AIKIDO"[TITLE/ABSTRACT] OR "WUSHU"[TITLE/ABSTRACT] OR "KUNG FU"[TITLE/ABSTRACT] OR "GONG FU"[TITLE/ABSTRACT] OR "GONGFU"[TITLE/ABSTRACT]))) OR (("SLEEP APNEA SYNDROMES"[MESH TERMS] OR "SLEEP APNEA*"[TITLE/ABSTRACT] OR "SLEEP HYPOPNEA"[TITLE/ABSTRACT] OR "SLEEP APNOEA*"[TITLE/ABSTRACT] OR "SLEEP DISORDERED BREATHING"[TITLE/ABSTRACT] OR ("POLYSOMNOGRAPHY"[MESH TERMS] OR "POLYSOMNOGRAPH*"[TITLE/ABSTRACT] OR "SLEEP STUDY"[TITLE/ABSTRACT] OR "SLEEP STUDIES"[TITLE/ABSTRACT] OR "SLEEP MONITOR*"[TITLE/ABSTRACT]) OR ("BARIUM SWALLOW"[TITLE/ABSTRACT] OR (("MANOMETRY"[MESH TERMS] OR "MANOMETR*"[TITLE/ABSTRACT]) AND ("ESOPHAG*"[TEXT WORD] OR "ESOPHOG*"[TITLE/ABSTRACT] OR "OROPHARYN*"[TEXT WORD])) OR ("ESOPHAGOGRAM*"[TITLE/ABSTRACT] OR "ESOPHOGRAPH*"[TITLE/ABSTRACT] OR "ESOPHAGRAPH*"[TITLE/ABSTRACT] OR "ESOPHAGRAM"[TITLE/ABSTRACT] OR "ESOPHOGRAM*"[TITLE/ABSTRACT] OR "PHARYNGOESOPHAGEAL MOTILITY STUD*"[TITLE/ABSTRACT] OR "CONTINUOUS ESOPHAGEAL PH MONITORING"[TITLE/ABSTRACT]) OR "VFSS"[TITLE/ABSTRACT] OR ("SWALLOW STUDY"[TITLE/ABSTRACT] OR "SWALLOW STUDIES"[TITLE/ABSTRACT] OR "SWALLOWING STUDY"[TITLE/ABSTRACT] OR "SWALLOWING STUDIES"[TITLE/ABSTRACT] OR "SWALLOWING EVALUATION*"[TITLE/ABSTRACT] OR "SWALLOW EVALUATION*"[TITLE/ABSTRACT]) OR ("DEGLUTITION DISORDERS"[MESH TERMS] OR "DEGLUTITION DISORDER*"[TITLE/ABSTRACT] OR "DYSPHAGIA*"[TITLE/ABSTRACT] OR "SWALLOWING DISORDER*"[TITLE/ABSTRACT] OR "SWALLOWING DYSFUNCTION*"[TITLE/ABSTRACT] OR "SWALLOWING IMPAIRMENT*"[TITLE/ABSTRACT]))) AND ((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE]) OR (("HETEROZYGOTE DETECTION*"[TITLE/ABSTRACT] OR "FAMILY"[TEXT WORD] OR "FAMILIAL"[TEXT WORD] OR "GENETIC CARRIER SCREENING"[MESH TERMS] OR "GENETIC*"[TITLE/ABSTRACT] OR "SIBLINGS"[MESH TERMS] OR "SIBLING*"[TITLE/ABSTRACT] OR "BROTHER*"[TITLE/ABSTRACT] OR "SISTER*"[TITLE/ABSTRACT] OR "FIRST DEGREE RELATIVE*"[TITLE/ABSTRACT] OR "PARENTS"[MESH TERMS] OR "PARENT*"[TITLE/ABSTRACT] OR "OFFSPRING"[TEXT WORD] OR "PEDIGREE"[MESH TERMS]) AND ((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE]) OR (((("CEREBELLAR TONSIL*"[TITLE/ABSTRACT] OR "CEREBELLAR VERMIS"[TEXT WORD]) AND ("REDUCTION*"[TITLE/ABSTRACT] OR "RESECTION*"[TITLE/ABSTRACT] OR "SHRINKAGE*"[TITLE/ABSTRACT] OR "SURGER*"[TEXT WORD] OR "NEUROSURG*"[TEXT WORD])) OR "TONSILLECTOMY"[MESH TERMS] OR "TONSILLECTOM*"[TITLE/ABSTRACT]) AND (((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE])) OR (((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE] AND ("INTRAOPERATIVE ELECTROPHYSIOLOGICAL MONITORING*"[TITLE/ABSTRACT] OR "INTRAOPERATIVE ELECTROPHYSIOLOGIC MONITORING*"[TITLE/ABSTRACT] OR (("MONITORING, INTRAOPERATIVE"[MESH TERMS] OR "INTRAOPERATIVE MONITOR*"[TITLE/ABSTRACT] OR "INTRA OPERATIVE MONITOR*"[TITLE/ABSTRACT]) AND ("NEURO*"[TEXT WORD] OR "BRAIN*"[TEXT WORD] OR "CEREBRAL*"[TEXT WORD] OR "CEREBELL*"[TEXT WORD])) OR ("NEUROMONITOR*"[TITLE/ABSTRACT] OR "NEURO MONITOR*"[TITLE/ABSTRACT] OR "BRAIN MONITORING*"[TITLE/ABSTRACT] OR "IONM"[TITLE/ABSTRACT] OR "CEREBRAL MONITORING*"[TITLE/ABSTRACT] OR "NEUROLOGIC MONITORING*"[TITLE/ABSTRACT] OR "NEUROLOGICAL MONITORING*"[TITLE/ABSTRACT] OR "INTRAOPERATIVE NEUROPHYSIOLOGICAL MONITORING"[MESH TERMS] OR "NEUROPHYSIOLOGICAL MONITORING*"[TITLE/ABSTRACT] OR "NEUROPHYSIOLOGIC MONITORING*"[TITLE/ABSTRACT] OR "TC MEP"[TITLE/ABSTRACT] OR "EVOKED POTENTIALS, MOTOR"[MESH TERMS] OR "EVOKED MOTOR POTENTIAL*"[TITLE/ABSTRACT] OR "MOTOR EVOKED POTENTIAL*"[TITLE/ABSTRACT] OR "SOMATOSENSORY EVOKED POTENTIAL*"[TITLE/ABSTRACT] OR "ULTRASONOGRAPHY, INTERVENTIONAL"[MESH TERMS:NOEXP] OR "INTERVENTIONAL ULTRASONOGRAPH*"[TITLE/ABSTRACT] OR "INTERVENTIONAL ULTRASOUND"[TITLE/ABSTRACT] OR "INTRAOPERATIVE USG"[TITLE/ABSTRACT] OR "INTRAOPERATIVE ULTRASO*"[TITLE/ABSTRACT]))) OR (("SYRINGOMYELIA"[MESH TERMS] OR "SYRINGOMYELIA*"[TEXT WORD] OR "SYRINGES"[TITLE/ABSTRACT] OR "SYRINX*"[TITLE/ABSTRACT]) AND ("REOPERATION"[MESH TERMS] OR "REOPERAT*"[TITLE/ABSTRACT] OR "REPEAT SURGER*"[TITLE/ABSTRACT] OR "REPEAT DECOMPRESSION"[TITLE/ABSTRACT] OR "REVISION SURGER*"[TITLE/ABSTRACT] OR "REINTERVEN*"[TITLE/ABSTRACT] OR "SECOND SURGER*"[TITLE/ABSTRACT] OR "SECONDARY SURGER*"[TITLE/ABSTRACT] OR "SECOND DECOMPRESSION*"[TITLE/ABSTRACT] OR "SECONDARY DECOMPRESSION*"[TITLE/ABSTRACT] OR "ADJUNCTIVE SURGER*"[TITLE/ABSTRACT] OR "SECONDARY PREVENTION"[MESH TERMS]) AND ((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE]) OR (("PFDD"[TITLE/ABSTRACT] OR "PFD"[TITLE/ABSTRACT] OR "PFDRT"[TITLE/ABSTRACT] OR "PFBD"[TITLE/ABSTRACT] OR "PFBDD"[TITLE/ABSTRACT] OR "DURA MATER/SURGERY"[MESH TERMS] OR "DURA SPLITTING*"[TITLE/ABSTRACT] OR "DURA MATER SURGER*"[TITLE/ABSTRACT] OR "DURAPLAST*"[TITLE/ABSTRACT] OR "DURA MATER TRANSPLANT*"[TITLE/ABSTRACT] OR "DURA MATER/TRANSPLANTATION"[MESH TERMS] OR "DURAL GRAFT*"[TITLE/ABSTRACT] OR "DURAL SUBSTITUTE*"[TITLE/ABSTRACT] OR "AUTOLOGOUS GRAFT*"[TITLE/ABSTRACT] OR "NONAUTOLOGOUS GRAFT*"[TITLE/ABSTRACT] OR "DURASEAL"[TITLE/ABSTRACT] OR "DUREPAIR"[TITLE/ABSTRACT] OR "ENDURA"[TITLE/ABSTRACT] OR "CADAVERIC PERICARDIUM"[TITLE/ABSTRACT] OR "AUTOGRAFTS"[MESH TERMS] OR "AUTOGRAFT*"[TITLE/ABSTRACT] OR "ALLOGRAFTS"[MESH TERMS] OR "ALLOGRAFT*"[TITLE/ABSTRACT] OR "DECOMPRESSION, SURGICAL"[MESH TERMS:NOEXP] OR "DECOMPRESSIVE CRANIECTOMY"[MESH TERMS] OR "CRANIAL FOSSA, POSTERIOR/SURGERY"[MESH TERMS] OR "BONY DECOMPRESSION*"[TITLE/ABSTRACT] OR "BONE ONLY DECOMPRESSION"[TITLE/ABSTRACT] OR "FORAMEN MAGNUM/SURGERY"[MESH TERMS] OR "FORAMEN MAGNUM DECOMPRESSION"[TITLE/ABSTRACT] OR "FORAMEN MAGNUM SURGER*"[TITLE/ABSTRACT] OR "OCCIPITOCERVICAL FIXATION*"[TITLE/ABSTRACT] OR "ATLANTO OCCIPITAL JOINT/SURGERY"[MESH TERMS] OR "OCCIPITOCERVICAL FUSION*"[TITLE/ABSTRACT] OR (("DECOMPRESSION"[TITLE/ABSTRACT] OR "DECOMPRESSIVE"[TITLE/ABSTRACT]) AND ("FOSSA CRANII POSTERIOR"[TITLE/ABSTRACT] OR "FOSSA POSTERIOR"[TITLE/ABSTRACT] OR "POSTERIOR CEREBRAL FOSSA*"[TITLE/ABSTRACT] OR "CRANIAL FOSSA, POSTERIOR"[MESH TERMS] OR "POSTERIOR CRANIAL FOSSA*"[TITLE/ABSTRACT] OR "POSTERIOR FOSSA*"[TITLE/ABSTRACT] OR "CLIVUS"[TITLE/ABSTRACT])) OR ("SURGER*"[TEXT WORD] OR "SURGICAL*"[TEXT WORD] OR "NEUROSURG*"[TEXT WORD] OR "NEURO SURG*"[TITLE/ABSTRACT])) AND ("DUTY TO RECONTACT"[MESH TERMS] OR "FOLLOW UP"[TITLE/ABSTRACT] OR "FOLLOW UPS"[TITLE/ABSTRACT] OR "FOLLOWS UP"[TITLE/ABSTRACT] OR "FOLLOWUP*"[TITLE/ABSTRACT] OR "RECONTACT*"[TITLE/ABSTRACT]) AND ((("ARNOLD CHIARI MALFORMATION"[MESH TERMS] OR "CHIARI*"[TEXT WORD]) NOT ("ANIMAL*"[MESH TERMS] NOT ("ANIMAL*"[MESH TERMS] AND "HUMAN*"[MESH TERMS]))) NOT ("LETTER"[PUBLICATION TYPE] OR "COMMENT"[PUBLICATION TYPE] OR "EDITORIAL"[PUBLICATION TYPE])) AND "ENGLISH"[LANGUAGE])
CHIARI MALFORMATION I – EMBASE SEARCH STRATEGY
(((THORACIC* OR THORACAL) NEAR/2 IMAGING*) OR (LUMBAR* NEAR/2 IMAGING*) OR (('THORACIC VERTEBRA'/EXP OR 'THORACIC VERTEBRAE':TI,AB OR 'THORACIC VERTEBRA':TI,AB OR 'THORACIC SPINE':TI,AB OR 'THORACIC VERTEBRAL':TI,AB OR 'THORAX SPINE':TI,AB OR 'LUMBAR VERTEBRAE'/EXP OR 'LUMBAR VERTEBRA':TI,AB OR 'LUMBAR VERTEBRAE':TI,AB OR 'LUMBAR SPINE*':TI,AB OR 'LUMBAR VERTEBRAL':TI,AB OR 'VERTEBRAE LUMBALES':TI,AB) AND ('DIAGNOSTIC IMAGING'/DE OR 'DIAGNOSTIC IMAGING':TI,AB OR 'NUCLEAR MAGNETIC RESONANCE IMAGING'/EXP OR 'MAGNETIC RESONANCE':TI,AB OR 'MAGNETIC RESONANCE TOMOGRAPHY':TI,AB OR 'MRI':TI,AB OR 'MRIS':TI,AB OR 'MR TOMOGRAPH*':TI,AB OR 'NMR TOMOGRAPH*':TI,AB OR 'ZEUGMATOGRAPH*':TI,AB OR 'PROTON SPIN TOMOGRAPH*':TI,AB OR 'FMRI':TI,AB)) OR (('GLIOMA'/EXP OR GLIOMA:TI,AB OR GANGLIOGLIOMA:TI,AB OR 'GLIA TUMOR':TI,AB OR 'GLIA TUMOUR':TI,AB OR 'GLIAL TUMOR':TI,AB OR 'GLIAL TUMOUR':TI,AB OR 'GLIAL CELL TUMOR':TI,AB OR 'GLIAL CELL TUMOUR':TI,AB OR 'BRAIN NEOPLASM':TI,AB OR 'BRAIN LESION':TI,AB OR 'BRAIN TUMOR*':TI,AB OR 'BRAIN TUMOUR*':TI,AB OR 'BRAIN CANCER*':TI,AB OR 'BRAIN TUMOR'/EXP OR 'CEREBRAL TUMOR':TI,AB OR 'CEREBRAL TUMOUR':TI,AB OR CEREBROMA:TI,AB OR 'CEREBRUM TUMOR':TI,AB OR 'CEREBRUM TUMOUR':TI,AB OR ENCEPHALOPHYMA:TI,AB OR 'INTRACEREBRAL TUMOR':TI,AB OR 'INTRACEREBRAL TUMOUR':TI,AB OR 'INTRACRANIAL NEOPLASM':TI,AB OR 'SUBTENTORIAL TUMOR':TI,AB OR 'SUBTENTORIAL TUMOUR':TI,AB OR 'SUPRATENTORIAL NEOPLASMS':TI,AB OR 'SUPRATENTORIAL TUMOR':TI,AB OR 'SUPRATENTORIAL TUMOUR':TI,AB OR 'TUMOR CEREBRI':TI,AB OR 'TUMOUR CEREBRI':TI,AB OR ((CEREBELLUM* OR CEREBELLAR* OR TONSIL*) NEAR/2 (TUMOR* OR TUMOUR* OR CANCER* OR NEOPLASM*)) OR 'HYDROCEPHALUS'/EXP OR HYDROCEPHAL*:TI,AB OR 'CEREBRAL VENTRICULOMEGALY':TI,AB OR 'AQUEDUCTAL STENOSIS':TI,AB,DE OR 'SYRINGOMYELIA'/EXP OR SYRINGOMYELIA*:TI,AB OR SYRINGOMYELUS*:TI,AB OR MYELOSYRINGOS*:TI,AB OR 'MORVAN DISEASE':TI,AB OR 'MORVAN S DISEASE':TI,AB OR HYDROSYRINGOMYELIA*:TI,AB OR SYRINX*:TI,AB OR 'NEURAL TUBE DEFECT'/EXP OR 'NEURAL TUBE DEFECT':TI,AB OR DYSRAPHIA:TI,AB OR 'DYSRAPHIC ANOMALIES':TI,AB OR 'DYSRAPHIC ANOMALY':TI,AB OR 'DYSRAPHIC STATE':TI,AB OR DYSRAPHISM:TI,AB OR DYSRAPHY:TI,AB OR 'NEURAL TUBE CLOSURE DEFECT':TI,AB OR 'NEURAL TUBE CLOSURE DEFECTS':TI,AB OR 'NEURAL TUBE DEFECTS':TI,AB OR 'NEURAL TUBE MALFORMATION':TI,AB OR 'TETHERED CORD SYNDROME'/EXP OR 'TETHERED SPINAL CORD':TI,AB OR 'TETHERING CORD':TI,AB OR 'TETHERED CORD':TI,AB OR 'SYRINX'/EXP) AND ('DIAGNOSTIC IMAGING'/DE OR 'DIAGNOSTIC IMAGING':TI,AB OR 'NUCLEAR MAGNETIC RESONANCE IMAGING'/EXP OR 'MAGNETIC RESONANCE':TI,AB OR 'MAGNETIC RESONANCE TOMOGRAPHY':TI,AB OR 'MRI':TI,AB OR 'MRIS':TI,AB OR 'MR TOMOGRAPH*':TI,AB OR 'NMR TOMOGRAPH*':TI,AB OR 'ZEUGMATOGRAPH*':TI,AB OR 'PROTON SPIN TOMOGRAPH*':TI,AB OR 'FMRI':TI,AB OR IMAGING*:TI,AB,DE)) OR (('IMAGING'/EXP OR IMAGING*:TI,AB OR 'NUCLEAR MAGNETIC RESONANCE IMAGING'/EXP OR 'MAGNETIC RESONANCE':TI,AB,DE OR 'MRI':TI,AB OR 'MRIS':TI,AB OR 'MR TOMOGRAPHY':TI,AB OR 'NMR TOMOGRAPHY':TI,AB OR 'ZEUGMATOGRAPHY':TI,AB OR 'PROTON SPIN TOMOGRAPHY':TI,AB OR 'FMRI':TI,AB OR 'CINE MAGNETIC RESONANCE IMAGING'/EXP OR 'CINE MAGNETIC RESONANCE IMAGING':TI,AB OR 'CINE-MRI':TI,AB OR 'CINE FLOW':TI,AB OR '4 D FLOW':TI,AB OR 'CINE MRI':TI,AB OR 'CINE MRIS':TI,AB) AND ('RANGE OF MOTION'/EXP OR 'RANGE OF MOTION':TI,AB,DE OR 'JOINT MOBILITY'/EXP OR 'JOINT MOBILITY':TI,AB OR 'JOINT FLEXIBILITY':TI,AB OR 'JOINT MOTILITY':TI,AB OR 'FLEXION'/EXP OR 'FLEXION':TI,AB OR 'EXTENSION'/EXP OR EXTENSION:TI,AB)) OR ((RADIOGRAPH*:TI,AB OR 'RADIOGRAPHY'/DE OR 'X RAY':TI,AB OR 'X-RAY':TI,AB OR XRAY*:TI,AB OR 'NEURORADIOLOGY'/EXP OR NEURORADIOLOG*:TI,AB OR NEURORADIOGRAPH*:TI,AB OR 'NEURO RADIOGRAPHY':TI,AB OR 'NEURO-RADIOGRAPHY':TI,AB OR 'NEURO RADIOLOGY':TI,AB OR 'NEURO-RADIOLOGY':TI,AB OR 'RADIOLOGY'/EXP OR RADIOLOG*:TI,AB OR ROENTGENOGRAPH*:TI,AB OR IMAGING*:TI,AB,DE) AND ('RANGE OF MOTION'/EXP OR 'RANGE OF MOTION':TI,AB OR 'FLEXION'/EXP OR 'FLEXION':TI,AB OR 'EXTENSION'/EXP OR 'EXTENSION':TI,AB OR 'HYPEREXTENSION'/EXP OR 'HYPEREXTENSION':TI,AB)) OR 'BASILAR IMPRESSION'/EXP OR 'BASILAR IMPRESSION':TI,AB OR 'BASILAR INVAGINATION':TI,AB OR 'BASILAR SKULL INVAGINATION':TI,AB OR PLATYBASIA*:TI,AB OR 'SKULL BASE INVAGINATION':TI,AB OR 'EHLERS DANLOS SYNDROME'/EXP OR 'EHLERS DANLOS':TI,AB OR 'EHLERS-DANLOS':TI,AB OR 'CUTIS LAXA'/EXP OR 'CUTIS LAXA':TI,AB OR 'CUTIS ELASTICA':TI,AB OR CHALASODERMA*:TI,AB OR CHALAZODERMA*:TI,AB OR CHALODERMA*:TI,AB OR 'CUTIS HYPERELASTICA':TI,AB OR 'CUTIS PENDULA':TI,AB OR 'ELASTIC FIBRODYSPLASIA':TI,AB OR 'ELASTIC SKIN':TI,AB OR 'FIBRODYSPLASIA ELASTICA':TI,AB OR 'INDIA RUBBER MAN':TI,AB OR 'EDS IV':TI,AB OR 'NECK INSTABILITY':TI,AB OR 'CERVICAL INSTABILITY'/EXP OR 'CERVICAL INSTABILITY':TI,AB OR 'CVJ INSTABILITY':TI,AB OR 'CRANIO-VERTEBRAL INSTABILITY':TI,AB OR 'CRANIOVERTEBRAL INSTABILITY':TI,AB OR 'CRANIO-VERTEBRAL JUNCTION INSTABILITY':TI,AB OR 'CRANIOVERTEBRAL JUNCTION INSTABILITY':TI,AB OR 'CRANIOCERVICAL INSTABILITY'/EXP OR 'CRANIOCERVICAL INSTABILITY':TI,AB OR 'CRANIO CERVICAL INSTABILITY':TI,AB OR 'CRANIAL CERVICAL INSTABILITY':TI,AB OR 'OBEX'/EXP OR OBEX:TI,AB OR 'CRANIOCERVICAL JUNCTION'/EXP OR 'CRANIOCERVICAL JUNCTION':TI,AB OR 'CRANIO CERVICAL JUNCTION':TI,AB OR (('CERVICAL VERTEBRA'/EXP OR 'CERVICAL VERTEBRAE':TI,AB OR 'CERVICAL VERTEBRA':TI,AB OR 'CERVICAL SPINE':TI,AB OR 'CERVICAL ATLAS':TI,AB OR 'C1 VERTEBRA':TI,AB OR 'ARCUATE FORAMEN':TI,AB OR 'PONTICULUS POSTICUS':TI,AB OR 'KIMMERLE ANOMALY':TI,AB OR 'PONTICULUS POSTERIOR OF THE ATLAS':TI,AB OR 'ODONTOID PROCESS':TI,AB OR 'DENS AXIS':TI,AB OR 'C2 VERTEBRA':TI,AB OR 'EPISTROPHEUS':TI,AB OR 'OS ODONTOIDEUM':TI,AB OR 'CRANIO-CERVICAL':TI,AB OR 'CRANIOCERVICAL':TI,AB OR 'CRANIAL-CERVICAL':TI,AB OR 'ARCUATE FORAMEN'/EXP OR 'PONTICULUS POSTICUS'/EXP OR 'ODONTOID PROCESS'/EXP OR 'ATLANTOAXIAL JOINT'/EXP OR 'ATLANTODENTAL JOINT':TI,AB OR 'ATLANTOOCCIPITAL JOINT'/EXP OR 'ATLANTO-AXIAL':TI,AB OR 'ATLANTOAXIAL':TI,AB OR 'ATLANTO-OCCIPITAL':TI,AB OR ATLANTOOCCIPITAL:TI,AB OR 'ATLOIDO OCCIPITAL JOINT':TI,AB OR 'OCCIPITOATLANTOAXIAL':TI,AB OR 'OCCIPITOCERVICAL':TI,AB OR 'OCCIPITAL CERVICAL':TI,AB) AND ('HYPERMOBILITY'/EXP OR HYPERMOBILIT*:TI,AB OR 'INSTABILITY'/EXP OR 'INSTABILITY':TI,AB OR UNSTABLE:TI,AB)) OR (('BRAIN STEM'/EXP OR 'BRAIN STEM':TI,AB OR BRAINSTEM*:TI,AB OR 'TRUNCUS CEREBI':TI,AB) AND 'COMPRESSION':TI,AB,DE) OR 'VENTRAL COMPRESSION':TI,AB OR 'VBSC':TI,AB OR 'CLIVUS-AXIS':TI,AB OR CLIVAL:TI,AB OR 'PB-C2':TI,AB OR 'CLIVOAXIAL ANGLE':TI,AB OR 'CLIVO AXIAL ANGLE':TI,AB OR CXA:TI,AB OR (('DECOMPRESSION SURGERY'/DE OR 'DECOMPRESSION SURGERY':TI,AB OR 'SURGICAL DECOMPRESSION':TI,AB OR 'DECOMPRESSION OPERATION':TI,AB OR 'DECOMPRESSIVE SURGERY':TI,AB OR ('DURA MATER' NEAR/2 (SURG* OR TRANSPLANT*)) OR 'DECOMPRESSIVE CRANIECTOMY'/EXP OR 'DECOMPRESSIVE CRANIECTOMY':TI,AB OR 'DECOMPRESSION CRANIECTOMY':TI,AB OR 'DECOMPRESSION CRANIOTOMY':TI,AB OR 'DECOMPRESSIVE CRANIOTOMY':TI,AB OR (('POSTERIOR FOSSA' OR 'FOSSA CRANII POSTERIOR' OR 'FOSSA POSTERIOR' OR 'POSTERIOR CEREBRAL FOSSA' OR 'POSTERIOR CRANIAL FOSSA' OR 'POSTERIOR SKULL FOSSA' OR 'POSTERIOR SKULL GROOVE') NEAR/2 (SURG* OR DECOMPRESS*)) OR ('FORAMEN MAGNUM' NEAR/2 (SURG* OR DECOMPRESS*)) OR (('ATLANTO OCCIPITAL' OR 'ATLANTOOCCIPITAL' OR 'ATLANTO-OCCIPITAL') NEAR/2 SURG*) OR 'OCCIPITOCERVICAL FIXATION'/EXP OR 'OCCIPITOCERVICAL FIXATION':TI,AB OR 'OCCIPITOCERVICAL FUSION'/EXP OR 'OCCIPITOCERVICAL FUSION':TI,AB OR 'PFDD':TI,AB OR 'PFD':TI,AB OR 'PFDRT':TI,AB OR 'PFBD':TI,AB OR 'PFBDD':TI,AB OR NEUROSURG*:TI,AB OR 'NEUROSURGERY'/DE OR 'NEUROLOGIC SURGERY':TI,AB OR 'NEUROLOGICAL SURGERY':TI,AB OR 'SURGERY':TI,AB,DE OR SURGICAL*:TI,AB OR 'DURA SPLITTING':TI,AB OR 'DURAPLASTY'/EXP OR DURAPLAST*TI,AB OR 'BONY DECOMPRESSION':TI,AB OR 'BONE ONLY DECOMPRESSION':TI,AB OR (('DECOMPRESSION':TI,AB OR 'DECOMPRESSIVE':TI,AB) AND ('CLIVUS':TI,AB OR 'DURAL SUBSTITUTE':TI,AB OR 'AUTOLOGOUS GRAFT':TI,AB OR 'NONAUTOLOGOUS GRAFT':TI,AB OR 'NON-AUTOLOGOUS GRAFT':TI,AB OR 'DURAL GRAFT':TI,AB OR 'DURASEAL':TI,AB OR 'DUREPAIR':TI,AB OR 'ENDURA':TI,AB OR 'CADAVERIC PERICARDIUM':TI,AB OR 'AUTOGRAFTS':TI,AB,DE OR 'AUTOGRAFT':TI,AB,DE OR 'ALLOGRAFTS':TI,AB,DE OR 'ALLOGRAFT':TI,AB,DE))) AND ('TREATMENT OUTCOME'/DE OR OUTCOME*:TI,AB,DE OR 'SURGICAL OUTCOME'/EXP OR 'TREATMENT FAILURE'/DE OR 'TREATMENT FAILURE':TI,AB OR 'THERAPY FAILURE':TI,AB OR ((IMPROV*:TI,AB OR RESOLUTION*:TI,AB OR RESOLV*:TI,AB OR EFFICAC*:TI,AB OR EFFECTIV*:TI,AB OR POST) AND OP*:TI,AB,DE))) OR (('ASYMPTOMATIC DISEASE'/EXP OR ASYMPTOMATIC*:TI,AB OR BENIGN*:TI,AB OR PRESYMPTOMATIC*:TI,AB OR 'PRE SYMPTOMATIC':TI,AB) AND ('DECOMPRESSION SURGERY'/DE OR 'DECOMPRESSION SURGERY':TI,AB OR 'SURGICAL DECOMPRESSION':TI,AB OR 'DECOMPRESSION OPERATION':TI,AB OR 'DECOMPRESSIVE SURGERY':TI,AB OR ('DURA MATER' NEAR/2 (SURG* OR TRANSPLANT*)) OR 'DECOMPRESSIVE CRANIECTOMY'/EXP OR 'DECOMPRESSIVE CRANIECTOMY':TI,AB OR 'DECOMPRESSION CRANIECTOMY':TI,AB OR 'DECOMPRESSION CRANIOTOMY':TI,AB OR 'DECOMPRESSIVE CRANIOTOMY':TI,AB OR (('POSTERIOR FOSSA' OR 'FOSSA CRANII POSTERIOR' OR 'FOSSA POSTERIOR' OR 'POSTERIOR CEREBRAL FOSSA' OR 'POSTERIOR CRANIAL FOSSA' OR 'POSTERIOR SKULL FOSSA' OR 'POSTERIOR SKULL GROOVE') NEAR/2 (SURG* OR DECOMPRESS*)) OR ('FORAMEN MAGNUM' NEAR/2 (SURG* OR DECOMPRESS*)) OR (('ATLANTO OCCIPITAL' OR 'ATLANTOOCCIPITAL' OR 'ATLANTO-OCCIPITAL') NEAR/2 SURG*) OR 'OCCIPITOCERVICAL FIXATION'/EXP OR 'OCCIPITOCERVICAL FIXATION':TI,AB OR 'OCCIPITOCERVICAL FUSION'/EXP OR 'OCCIPITOCERVICAL FUSION':TI,AB OR 'PFDD':TI,AB OR 'PFD':TI,AB OR 'PFDRT':TI,AB OR 'PFBD':TI,AB OR 'PFBDD':TI,AB OR NEUROSURG*:TI,AB OR 'NEUROSURGERY'/DE OR 'NEUROLOGIC SURGERY':TI,AB OR 'NEUROLOGICAL SURGERY':TI,AB OR 'SURGERY':TI,AB,DE OR SURGICAL*:TI,AB OR 'DURA SPLITTING':TI,AB OR 'DURAPLASTY'/EXP OR DURAPLAST*TI,AB OR 'BONY DECOMPRESSION':TI,AB OR 'BONE ONLY DECOMPRESSION':TI,AB OR (('DECOMPRESSION':TI,AB OR 'DECOMPRESSIVE':TI,AB) AND ('CLIVUS':TI,AB OR 'DURAL SUBSTITUTE':TI,AB OR 'AUTOLOGOUS GRAFT':TI,AB OR 'NONAUTOLOGOUS GRAFT':TI,AB OR 'NON-AUTOLOGOUS GRAFT':TI,AB OR 'DURAL GRAFT':TI,AB OR 'DURASEAL':TI,AB OR 'DUREPAIR':TI,AB OR 'ENDURA':TI,AB OR 'CADAVERIC PERICARDIUM':TI,AB OR 'AUTOGRAFTS':TI,AB,DE OR 'AUTOGRAFT':TI,AB,DE OR 'ALLOGRAFTS':TI,AB,DE OR 'ALLOGRAFT':TI,AB,DE)) OR 'PROPHYLACTIC SURGICAL PROCEDURE' OR PROPHYLACTIC*:TI,AB OR 'CONSERVATIVE TREATMENT':TI,AB OR 'CONSERVATIVE TREATMENT'/DE OR 'CONSERVATIVE MANAGEMENT':TI,AB OR 'CONSERVATIVE THERAPY':TI,AB OR 'NONOPERATIVE TREATMENT':TI,AB OR 'NONSURGICAL TREATMENT':TI,AB OR 'ORGAN SPARING TREATMENT':TI,AB OR 'ORGAN SPARING TREATMENTS':TI,AB OR 'CLINICAL DECISION MAKING'/EXP OR 'CLINICAL DECISION MAKING':TI,AB OR 'WATCHFUL WAITING'/EXP OR 'WATCHFUL WAITING':TI,AB OR 'ACTIVE SURVEILLANCE'/EXP OR 'ACTIVE SURVEILLANCE':TI,AB OR 'WAIT AND SEE':TI,AB OR 'EXPECTANT MANAGEMENT'/EXP OR 'EXPECTANT MANAGEMENT':TI,AB)) OR 'ACTIVITY RESTRICTION'/EXP OR 'ACTIVITY RESTRICTION':TI,AB OR 'ACTIVITY RESTRICTIONS':TI,AB OR 'SPORT'/EXP OR SPORT*:TI,AB,DE OR GYMNASTICS:TI,AB OR 'ATHLETE'/EXP OR ATHLETIC*:TI,AB OR ATHLETE*:TI,AB OR 'FOOTBALL'/EXP OR 'FOOTBALL':TI,AB,DE OR 'RUGBY'/EXP OR 'RUGBY':TI,AB,DE OR 'FOOTBALL PLAYER' OR 'SOCCER'/EXP OR 'SOCCER':TI,AB,DE OR 'BOXING'/EXP OR 'BOXING':TI,AB,DE OR 'WRESTLING'/EXP OR 'WRESTLING':TI,AB OR WRESTLER*:TI,AB OR 'WEIGHT LIFTING'/EXP OR 'WEIGHT LIFTING':TI,AB OR 'WEIGHT LIFTER':TI,AB OR 'ICE HOCKEY'/EXP OR HOCKEY*:TI,AB OR 'LACROSSE'/EXP OR 'LACROSSE':TI,AB OR 'HURLING'/EXP OR 'HURLING':TI,AB OR 'FUTSAL'/EXP OR 'FUTSAL':TI,AB OR 'WATER POLO'/EXP OR 'WATER POLO':TI,AB OR WATERPOLO:TI,AB OR 'ROLLER DERBY':TI,AB OR KABADI:TI,AB OR KABADDI:TI,AB OR 'BASKETBALL'/EXP OR 'BASKETBALL':TI,AB OR 'BASKET BALL':TI,AB OR QUIDDITCH:TI,AB OR SHINTY:TI,AB OR 'MARTIAL ART'/EXP OR 'MARTIAL ART':TI,AB OR 'MARTIAL ARTS':TI,AB OR 'JUDO':TI,AB OR 'KARATE':TI,AB OR KARATEKA*:TI,AB OR JUJITSU:TI,AB OR 'TAEKWONDO':TI,AB OR 'TAE KWON DO':TI,AB OR 'AIKIDO':TI,AB OR WUSHU:TI,AB OR 'KUNG FU':TI,AB OR 'GONG FU':TI,AB OR GONGFU:TI,AB OR 'SLEEP DISORDERED BREATHING'/EXP OR 'SLEEP DISORDERED BREATHING':TI,AB OR 'SLEEP APNEA':TI,AB OR 'NOCTURNAL APNEA':TI,AB OR 'NOCTURNAL APNOEA':TI,AB OR 'SLEEP APNOEA':TI,AB OR 'SLEEP HYPOPNEA':TI,AB OR 'POLYSOMNOGRAPHY'/EXP OR POLYSOMNOGRAPH*:TI,AB OR 'SLEEP STUDY':TI,AB OR 'SLEEP STUDIES':TI,AB OR 'SLEEP MONITORING':TI,AB OR (('MANOMETRY'/EXP OR MANOMETR*:TI,AB,DE) AND ('ESOPHAGUS'/EXP OR ESOPHAG*:TI,AB,DE OR OESOPHAG*:TI,AB OR 'OROPHARYNX'/EXP OR OROPHARYN*:TI,AB)) OR 'DYSPHAGIA'/EXP OR DYSPHAGIA*:TI,AB OR 'DEGLUTITION DISORDER':TI,AB OR APHAGOPRAXIA*:TI,AB OR 'DEGLUTITION DIFFICULTY':TI,AB OR 'DIFFICULT DEGLUTITION':TI,AB OR 'DIFFICULTY SWALLOWING':TI,AB OR 'SWALLOWING DIFFICULTY':TI,AB OR 'SWALLOWING DISORDER':TI,AB OR 'SWALLOWING DYSFUNCTION':TI,AB OR 'SWALLOWING IMPAIRMENT':TI,AB OR 'ESOPHAGOGRAPHY'/EXP OR ESOPHAGOGRAPH*:TI,AB OR 'BARIUM SWALLOW':TI,AB OR ESOPHAGOGRAM*:TI,AB OR 'ESOPHAGUS RADIOGRAPHY':TI,AB OR OESOPHAGOGRAPH*:TI,AB OR 'PHARYNGOESOPHAGEAL MOTILITY STUDY':TI,AB OR 'PHARYNGOESOPHAGEAL MOTILITY STUDIES':TI,AB OR 'CONTINUOUS ESOPHAGEAL PH MONITORING':TI,AB OR VFSS:TI,AB OR 'SWALLOW STUDY':TI,AB OR 'SWALLOW STUDIES':TI,AB OR 'SWALLOWING STUDY':TI,AB OR 'SWALLOWING STUDIES':TI,AB OR 'SWALLOWING EVALUATION*':TI,AB OR 'SWALLOW EVALUATION*':TI,AB OR 'HETEROZYGOTE DETECTION'/EXP OR 'HETEROZYGOTE DETECTION':TI,AB OR 'GENETIC CARRIER SCREENING':TI,AB OR 'CARRIER DETECTION':TI,AB OR 'GENETIC CARRIER DETECTION':TI,AB OR 'HETEROZYGOTE SCREENING':TI,AB OR 'HETEROZYGOTE TEST':TI,AB OR 'HETEROZYGOTE TESTING':TI,AB OR 'SIBLING'/EXP OR SIBLING*:TI,AB OR BROTHER*:TI,AB OR SISTER*:TI,AB OR 'FIRST-DEGREE RELATIVE'/EXP OR 'FIRST-DEGREE RELATIVE':TI,AB OR '1ST DEGREE RELATIVE':TI,AB OR 'FIRST-DEGREE BLOOD RELATIVE':TI,AB OR 'PARENT'/EXP OR PARENT:TI,AB OR PARENTS:TI,AB OR 'PROGENY'/EXP OR 'PROGENY':TI,AB OR OFFSPRING:TI,AB OR 'FAMILIAL RISK'/EXP OR 'FAMILIAL RISK':TI,AB OR 'PEDIGREE'/EXP OR 'PEDIGREE':TI,AB OR 'FAMILIAL AGGREGATION'/EXP OR 'FAMILIAL AGGREGATION':TI,AB OR GENETIC*:TI,AB,DE OR 'FAMILY HISTORY'/EXP OR 'FAMILY HISTORY':TI,AB OR 'FAMILY MEDICAL HISTORY':TI,AB OR 'FAMILY ANAMNESIS':TI,AB OR (('CEREBELLAR TONSIL'/EXP OR 'CEREBELLAR TONSIL':TI,AB OR 'CEREBELLAR VERMIS':TI,AB) AND (NEUROSURG*:TI,AB,DE OR 'NEUROLOGIC SURGERY':TI,AB OR 'NEUROLOGICAL SURGERY':TI,AB OR 'NEUROSURGICAL PROCEDURE':TI,AB OR 'NEUROSURGICAL PROCEDURES':TI,AB OR 'SURGERY'/DE OR SURG*:TI,AB OR REDUCTION*:TI,AB OR RESECTION*:TI,AB OR SHRINKAGE*:TI,AB)) OR (('CEREBELLAR TONSIL'/EXP OR 'CEREBELLAR TONSIL':TI,AB OR 'CEREBELLAR VERMIS':TI,AB) AND ('TONSILLECTOMY'/EXP OR TONSILLECTOM*:TI,AB)) OR 'INTRAOPERATIVE ELECTROPHYSIOLOGICAL MONITORING':TI,AB OR 'INTRAOPERATIVE ELECTROPHYSIOLOGIC MONITORING':TI,AB OR (('INTRAOPERATIVE MONITORING'/EXP OR 'INTRASURGICAL MONITORING':TI,AB OR 'INTRAOPERATIVE MONITORING':TI,AB OR 'INTRA OPERATIVE MONITORING':TI,AB) AND (NEURO*:TI,AB,DE OR BRAIN*:TI,AB,DE OR CEREBRAL*:TI,AB,DE OR CEREBELL*:TI,AB,DE)) OR 'INTRAOPERATIVE NEUROPHYSIOLOGICAL MONITORING'/EXP OR 'NEUROPHYSIOLOGICAL INTRAOPERATIVE MONITORING':TI,AB OR 'INTRAOPERATIVE NEUROPHYSIOLOGICAL MONITORING':TI,AB,DE OR 'INTRAOPERATIVE NEUROPHYSIOLOGIC MONITORING':TI,AB,DE OR 'NEUROPHYSIOLOGICAL MONITORING'/EXP OR 'NEUROPHYSIOLOGICAL MONITORING':TI,AB OR 'NEUROPHYSIOLOGIC MONITORING':TI,AB OR 'TC-MEP':TI,AB OR 'MOTOR EVOKED POTENTIAL'/EXP OR 'MOTOR EVOKED POTENTIAL':TI,AB OR 'EVOKED MOTOR RESPONSE':TI,AB OR 'EVOKED MUSCLE RESPONSE':TI,AB OR 'EVOKED MUSCULAR RESPONSE':TI,AB OR 'EVOKED MOTOR POTENTIAL':TI,AB OR 'SOMATOSENSORY EVOKED POTENTIAL'/EXP OR 'SOMATOSENSORY EVOKED POTENTIAL':TI,AB OR 'EVOKED SOMAESTHETIC RESPONSE':TI,AB OR 'EVOKED SOMATO SENSORY RESPONSE':TI,AB OR 'EVOKED SOMATOSENSORY POTENTIAL':TI,AB OR 'EVOKED SOMATOSENSORY REPONSE':TI,AB OR 'EVOKED SOMATOSENSORY RESPONSE':TI,AB OR 'EVOKED SOMESTHETIC RESPONSE':TI,AB OR 'SOMATIC EVOKED RESPONSE':TI,AB OR 'SOMATO SENSORY EVOKED POTENTIAL':TI,AB OR 'SOMATO SENSORY EVOKED RESPONSE':TI,AB OR 'SOMATOSENSORY EVOKED POTENTIALS':TI,AB OR 'SOMATOSENSORY EVOKED RESPONSE':TI,AB OR SSER:TI,AB OR 'INTERVENTIONAL ULTRASONOGRAPHY'/EXP OR 'INTERVENTIONAL ULTRASONOGRAPHY':TI,AB OR 'ULTRASOUND-GUIDED INTERVENTION':TI,AB OR 'US-GUIDED INTERVENTION':TI,AB OR 'INTERVENTIONAL ULTRASOUND':TI,AB OR 'INTRAOPERATIVE USG':TI,AB OR 'INTRAOPERATIVE ULTRASOUND':TI,AB OR (('SYRINGOMYELIA'/EXP OR SYRINGOMYELIA*:TI,AB OR (MYELOSYRINGOS*:TI,AB AND SYRINGOHYDROMYELIA*:TI,AB) OR 'SYRINGOMYELIC SYNDROME':TI,AB OR SYRINGOMYELIN*:TI,AB OR SYRINGOMYELY:TI,AB OR 'SYRINX'/EXP OR SYRINX*:TI,AB OR SYRINGES:TI,AB) AND (SHRINK*:TI,AB OR REGRESS*:TI,AB OR REDUC*:TI,AB OR RESOLUTION*:TI,AB OR RESOLV*:TI,AB OR RECUR*:TI,AB OR NARROWING*:TI,AB OR DIMINISH*:TI,AB OR DECREAS*:TI,AB OR DISAPPEAR*:TI,AB OR REVERS*:TI,AB OR PERSIST*:TI,AB OR WORSEN*:TI,AB OR IMPROV*:TI,AB) AND ('REOPERATION'/EXP OR REOPERAT*:TI,AB OR (REPEAT NEAR/2 SURG*) OR (REPEAT NEAR/2 DECOMPRESSION*) OR (SECOND* NEAR/2 DECOMPRESSION*) OR (REVISION NEAR/2 SURG*) OR 'REINTERVENTION'/EXP OR REINTERVENTION*:TI,AB OR (SECOND* NEAR/2 SURG*) OR 'SECONDARY PREVENTION'/EXP OR 'SECONDARY PREVENTION':TI,AB OR (ADJUNCTIVE NEAR/2 SURG*))) OR (('SYRINGOMYELIA'/EXP OR SYRINGOMYELIA*:TI,AB OR (MYELOSYRINGOS*:TI,AB AND SYRINGOHYDROMYELIA*:TI,AB) OR 'SYRINGOMYELIC SYNDROME':TI,AB OR SYRINGOMYELIN*:TI,AB OR SYRINGOMYELY:TI,AB OR 'SYRINX'/EXP OR SYRINX*:TI,AB OR SYRINGES:TI,AB) AND ('REOPERATION'/EXP OR REOPERAT*:TI,AB OR (REPEAT NEAR/2 SURG*) OR (REPEAT NEAR/2 DECOMPRESSION*) OR (SECOND* NEAR/2 DECOMPRESSION*) OR (REVISION NEAR/2 SURG*) OR 'REINTERVENTION'/EXP OR REINTERVENTION*:TI,AB OR (SECOND* NEAR/2 SURG*) OR 'SECONDARY PREVENTION'/EXP OR 'SECONDARY PREVENTION':TI,AB OR (ADJUNCTIVE NEAR/2 SURG*)) AND ('TIME FACTOR'/EXP OR 'TIME FACTOR':TI,AB OR 'TIME FACTORS':TI,AB OR 'FOLLOW UP'/EXP OR 'FOLLOW UP':TI,AB OR 'TREATMENT OUTCOME'/EXP OR 'TREATMENT OUTCOME':TI,AB OR 'SURGICAL OUTCOME'/EXP OR 'SURGICAL OUTCOME':TI,AB OR 'TIME TO RESOLUTION':TI,AB OR 'TREATMENT RESPONSE TIME'/EXP OR 'TREATMENT RESPONSE TIME':TI,AB OR 'TIME TO RECURRENCE'/EXP OR 'TIME TO RECURRENCE':TI,AB OR 'TIME TO RELAPSE'/EXP OR 'TIME TO RELAPSE':TI,AB OR 'CHICAGO CHIARI OUTCOME SCALE'/EXP OR 'CHICAGO CHIARI OUTCOME':TI,AB)) OR (('DECOMPRESSION SURGERY'/DE OR 'DECOMPRESSION SURGERY':TI,AB OR 'SURGICAL DECOMPRESSION':TI,AB OR 'DECOMPRESSION OPERATION':TI,AB OR 'DECOMPRESSIVE SURGERY':TI,AB OR ('DURA MATER' NEAR/2 (SURG* OR TRANSPLANT*)) OR 'DECOMPRESSIVE CRANIECTOMY'/EXP OR 'DECOMPRESSIVE CRANIECTOMY':TI,AB OR 'DECOMPRESSION CRANIECTOMY':TI,AB OR 'DECOMPRESSION CRANIOTOMY':TI,AB OR 'DECOMPRESSIVE CRANIOTOMY':TI,AB OR (('POSTERIOR FOSSA' OR 'FOSSA CRANII POSTERIOR' OR 'FOSSA POSTERIOR' OR 'POSTERIOR CEREBRAL FOSSA' OR 'POSTERIOR CRANIAL FOSSA' OR 'POSTERIOR SKULL FOSSA' OR 'POSTERIOR SKULL GROOVE') NEAR/2 (SURG* OR DECOMPRESS*)) OR ('FORAMEN MAGNUM' NEAR/2 (SURG* OR DECOMPRESS*)) OR (('ATLANTO OCCIPITAL' OR 'ATLANTOOCCIPITAL' OR 'ATLANTO-OCCIPITAL') NEAR/2 SURG*) OR 'OCCIPITOCERVICAL FIXATION'/EXP OR 'OCCIPITOCERVICAL FIXATION':TI,AB OR 'OCCIPITOCERVICAL FUSION'/EXP OR 'OCCIPITOCERVICAL FUSION':TI,AB OR 'PFDD':TI,AB OR 'PFD':TI,AB OR 'PFDRT':TI,AB OR 'PFBD':TI,AB OR 'PFBDD':TI,AB OR NEUROSURG*:TI,AB OR 'NEUROSURGERY'/DE OR 'NEUROLOGIC SURGERY':TI,AB OR 'NEUROLOGICAL SURGERY':TI,AB OR 'SURGERY':TI,AB,DE OR SURGICAL*:TI,AB OR 'DURA SPLITTING':TI,AB OR 'DURAPLASTY'/EXP OR DURAPLAST*TI,AB OR 'BONY DECOMPRESSION':TI,AB OR 'BONE ONLY DECOMPRESSION':TI,AB OR (('DECOMPRESSION':TI,AB OR 'DECOMPRESSIVE':TI,AB) AND ('CLIVUS':TI,AB OR 'DURAL SUBSTITUTE':TI,AB OR 'AUTOLOGOUS GRAFT':TI,AB OR 'NONAUTOLOGOUS GRAFT':TI,AB OR 'NON-AUTOLOGOUS GRAFT':TI,AB OR 'DURAL GRAFT':TI,AB OR 'DURASEAL':TI,AB OR 'DUREPAIR':TI,AB OR 'ENDURA':TI,AB OR 'CADAVERIC PERICARDIUM':TI,AB OR 'AUTOGRAFTS':TI,AB,DE OR 'AUTOGRAFT':TI,AB,DE OR 'ALLOGRAFTS':TI,AB,DE OR 'ALLOGRAFT':TI,AB,DE))) AND ('DUTY TO RECONTACT'/EXP OR 'DUTY TO RECONTACT':TI,AB OR 'FOLLOW UP'/EXP OR 'FOLLOW UP':TI,AB OR AFTERCARE:TI,AB,DE OR FOLLOWUP*:TI,AB OR 'FOLLOWS UP':TI,AB OR 'FOLLOW UPS':TI,AB OR RECONTACT*:TI,AB))) AND ('ARNOLD CHIARI MALFORMATION'/EXP OR CHIARI*:TI,AB) NOT ('ANIMAL'/EXP NOT ('ANIMAL'/EXP AND 'HUMAN'/EXP)) NOT ('LETTER'/EXP OR 'EDITORIAL'/EXP OR 'CASE REPORT'/EXP) AND [ENGLISH]/LIM NOT 'CONFERENCE ABSTRACT'/EXP AND ('ARTICLE'/IT OR 'ARTICLE IN PRESS'/IT OR 'REVIEW'/IT)
Appendix II. Rating evidence quality
Classification of Evidence on Therapeutic Effectiveness and Levels of Recommendation
Class I evidence
Level I (or A) recommendation |
Evidence from one or more well-designed, randomized controlled clinical trial, including overviews of such trials |
Class II evidence
Level II (or B) recommendation |
Evidence from one or more well-designed comparative clinical studies, such as nonrandomized cohort studies, case-control studies, and other comparable studies, including less well-designed randomized controlled trials |
Class III evidence
Level III (or C) recommendation |
Evidence from case series, comparative studies with historical controls, case reports, and expert opinion, as well as significantly flawed randomized controlled trials |
Classification of Evidence on Prognosis and Levels of Recommendation
Class I evidence
Level I (or A) recommendation |
All 5 technical criteria above are satisfied |
Class II evidence
Level II (or B) recommendation |
Four of 5 technical criteria are satisfied |
Class III evidence
Level III (or C) recommendation |
Everything else |
Classification of Evidence on Diagnosis and Levels of Recommendation
Class I evidence
Level I (or A) recommendation |
Evidence provided by one or more well-designed clinical studies of a diverse population using a “gold standard” reference test in a blinded evaluation appropriate for the diagnostic applications and enabling the assessment of sensitivity, specificity, positive and negative predictive values, and, where applicable, likelihood ratios |
Class II evidence
Level II (or B) recommendation |
Evidence provided by one or more well-designed clinical studies of a restricted population using a “gold standard” reference test in a blinded evaluation appropriate for the diagnostic applications and enabling the assessment of sensitivity, specificity, positive and negative predictive values, and, where applicable, likelihood ratios |
Class III evidence
Level III (or C) recommendation |
Evidence provided by expert opinion or studies that do not meet the criteria for the delineation of sensitivity, specificity, positive and negative predictive values, and, where applicable, likelihood ratios |
Classification of Evidence on Clinical Assessment and Levels of Recommendation
Class I evidence
Level I (or A) recommendation |
Evidence provided by one or more well-designed clinical studies in which interobserver and/or intraobserver reliability is represented by a kappa statistic >0.60 |
Class II evidence
Level II (or B) recommendation |
Evidence provided by one or more well-designed clinical studies in which interobserver and/or intraobserver reliability is represented by a kappa statistic >0.40 |
Class III evidence
Level III (or C) recommendation |
Evidence provided by one or more well-designed clinical studies in which interobserver and/or intraobserver reliability is represented by a kappa statistic <0.40 |
Appendix III. PRISMA flowchart
PICO 1-1.
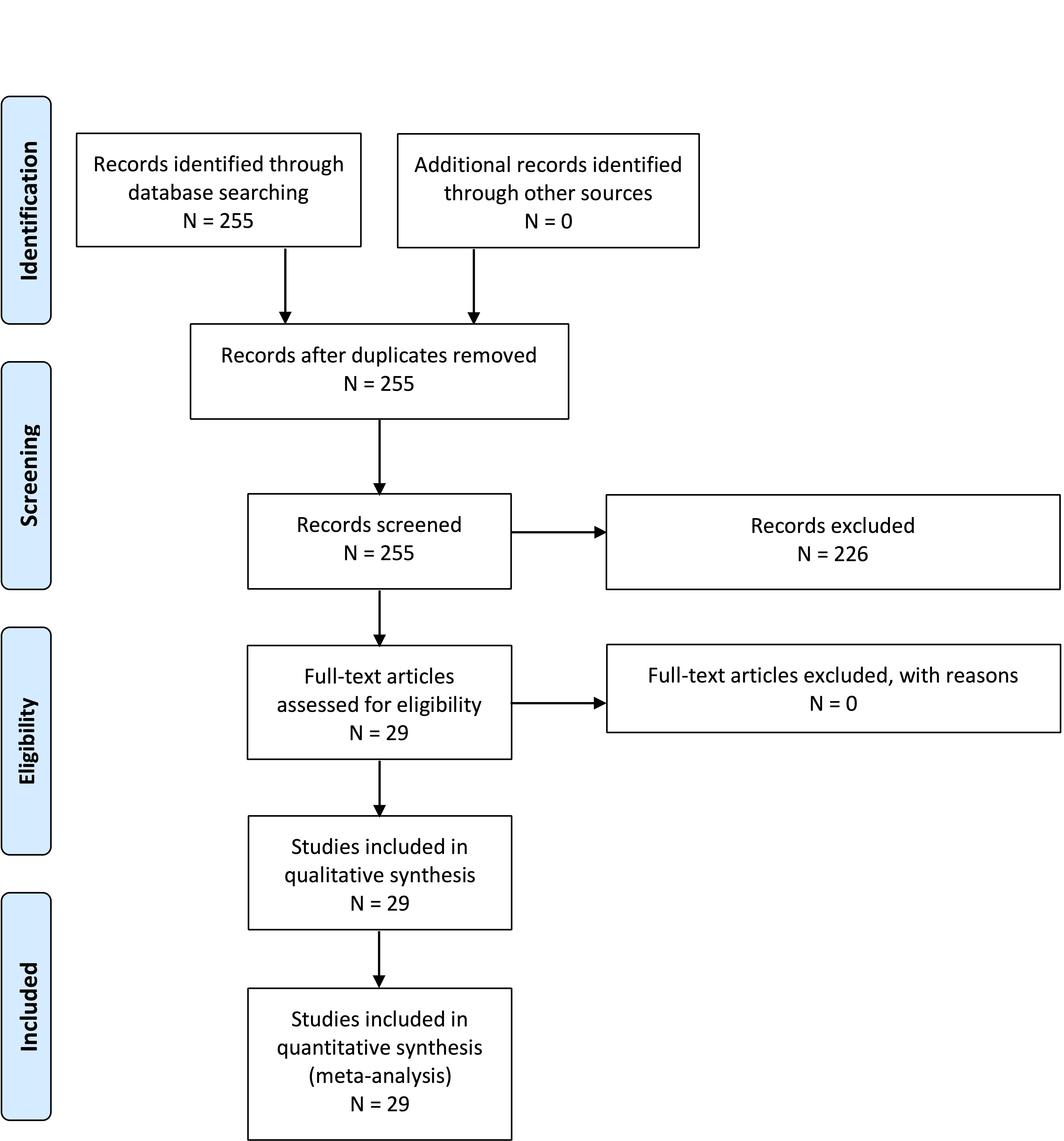
PICO 1-2.
PICO 1-3.
Appendix IV. Evidence tables
| PICO |
Author, Year |
Type of Evidence |
Study Type |
Class of Evidence |
Reviewer’s Conclusions |
| 1 |
Strahle et al, 20152 |
Patient assessment |
Retrospective case series |
III |
CIM may not be independently associated with scoliosis, and scoliosis may not be a symptom of CIM. The aim was to see if CIM is associated with scoliosis independent of syrinx; included patients: 509 Chiari, 1740 scoli, and 243 syrinx |
| 1 |
Milhorat et al, 20093 |
Therapy/patient assessment |
Retrospective case control |
III |
Determines if section film was beneficial for patients with CIM. Evaluates association between CIM and TCS. discusses sectioning of the filum terminal. found improved symptoms of TCS and improvement in the cerebellar tonsils |
| 1 |
Leung, V et al, 20164 |
Diagnostic |
Retrospective case control |
III |
Measures motion of cerebellum in Chiari/control patients; in patients with CIM and cardiac gated Cine MRI, cerebellar tonsil motion may be related to CIM; looks at the motion of the cerebellar tonsils in control patients, preop CIM patients with and without syringomyelia and postop patients. Includes Chiari decompression |
| 1 |
Taylor et al, 20205 |
Therapy/patient assessment |
Retrospective case series |
III |
Evaluated subarachnoid space at foramen magnum for syrinx resolution; shows correlation between syringogenesis and diminution of shubarachnoid space in patients with CIM; lack of follow-up |
| 1 |
Milhorat et al, 19996 |
Patient assessment |
Prospective case series |
III |
Chiari may be related to underdevelopment of posterior fossa; symptoms may be due to CSF flow blockage or direct compression; there may be a familial component for some patients; includes posterior fossa size and associated symptoms; includes information about the origins and etiology in patients with CIM |
| 1 |
Elster et al, 19927 |
Patient assessment |
Prospective case series |
III |
Correlate symptoms with radiographic findings; no treatment was included (observational study); cerebellar tonsillation and presence of a syrinx |
| 1 |
Bollo et al, 20128 |
Patient assessment |
Retrospective case series |
III |
Determines radiographic parameters needed from OC fusion; includes preoperative risk factors for patients with CIM that could potentially need occipitocervical fusion; Includes suboccipital decompression and or fusion; Shows need for fusion due to basilar invagination |
| 1 |
Tubbs et al, 20119 |
Patient assessment |
Retrospective case series |
III |
Patients with CIM might present with headache/ neck pain and scoliosis. Other imaging findings or symptoms are described. This is a retrospective view of 500 surgically treated patients; results include symptom improvement and resolution of syrinx |
| 1 |
Kennedy et al, 201610 |
Patient assessment |
Retrospective case series |
III |
Includes 12 patients with isolated thoracic syrinx, 50% syrinx; objective of this study was to determine the number of patients with a CIM that have an isolated thoracic syrinx; No specific treatments were identified; results include symptoms associated with isolated thoracic syrinx |
| 1 |
Tubbs et al, 200011 |
Patient assessment |
Retrospective case series |
III |
Evaluates conus position, syrinx, Chiari relationship; compares observational vs no treatment; to see whether there is a relationship between conus even and patients with a CIM; level of conus and syrinx |
| 1 |
Sadler et al, 202012 |
Patient assessment |
Retrospective case series |
III |
Identify underlying other DX with CIM to determine if other comorbidities are present in patients with CIM |
| 1 |
Menezes et al, 199513 |
Patient assessment |
Prospective case series |
III |
Identify ways to treat complex CIM including craniocervical fixation and fusion, various ways; posterior decompression and fusion; resolution of syringohydromelia and resterablishment of CSF flow |
| 1 |
Strahle et al, 202014 |
Patient assessment |
Retrospective/prospective case series |
III |
Determines associated factors between Chiari and scoliosis; determines the clinical and radiologic predictors of curve regression after PFD in patients with CIM; curve progression >5 degrees; regression of the scoliosis curve |
| 1 |
McGirt, et al, 200615 |
Patient assessment |
Retrospective case series |
III |
Association between Cine flow and improvement after Chiari decompression; explores whether or not CSF flow dynamics assessed by Cine phase contrast MRI could independently predict response to posterior fossa decompression for CIM; follow-up was 1 month and 1 year after surgery—no mean follow-up was included |
| 1 |
Caldarelli et al, 200716 |
Therapy/patient assessment |
Retrospective case series |
III |
Role of limited posterior fossa crani in CIM; determine if PFD works for CIM; extradural only procedure with C1 laminectomy |
| 1 |
Krieger et al, 201117 |
Patient assessment |
Retrospective case series |
III |
Evaluates effect of CIM decompression on scoliosis; to see the association between CIM and scoliosis in children; needing further orthopedic procedure to correct the curvature; craniocervical decompression in a standard fashion |
| 1 |
Brockmeyer et al 200318 |
Patient assessment |
Retrospective case series |
III |
Identifies relationship between CIM and scoliosis; effect of subocciptal decompression on curve progression; curve improvement or necessity of a fusion |
| 1 |
Bhangoo et al, 200619 |
Patient assessment |
Retrospective case series |
III |
Determines association between CIM and scoliosis; whether or not the scoliosis curve improved after suboccipital decompression |
| 1 |
Muhonen et al, 199220 |
Patient assessment |
Retrospective case series |
III |
Association between CIM and scoliosis; effect of treatment of CIM and scoliosis; PFDD, transoral, fusion; transoral and suboccipital decompression; population is heterogeneous |
| 1 |
O'Neill et al 202121 |
Patient assessment |
Retrospective case series |
III |
Chiari decompression in asymptomatic patient with scoliosis may not be helpful to change outcome of curve progression or need for spine fusion; association between CIM and scoliosis; scoliosis in patients with CIM without a syrinx; curve progression or stabilization |
| 1 |
Mauer et al 201122 |
Patient assessment |
Retrospective case series |
III |
CINE flow in patients pre- and postdecompression; demonstration of CSF pulsations can indicate surgical outcomes |
| 1 |
Fan et al, 201723 |
Patient assessment |
Retrospective case series |
III |
Evaluates blockage location and treatment for CIM with syrinx; different surgical approaches are hypothesized based on CSF flow dynamics; includes PFD and PFD subarachnoid manipulation; authors were subjective with respect to Cine imaging; population was heterogeneous |
| 1 |
Lee et al, 201424 |
Patient assessment |
Retrospective case series |
III |
Evaluation of optimal treatment of CIM; shows improvement in symptoms and syrinx |
| 1 |
Villa et al, 201925 |
Patient assessment |
Retrospective case series |
III |
Evaluates efficacy of surgery; shows improvement in symptoms and syrinx |
| 1 |
Menezes et al, 201826 |
Patient assessment |
Retrospective case series |
III |
Includes characteristics of CIM patients with syringobulbia; assesses if the syringobulbia resolved after PFD in patients with CIM; results show that in patients with syringobulbia and Chiari, posterior fossa decompression with intradural exploration and duraplasty may treat symptoms and imaging |
| 1 |
Gad et al, 201727 |
Patient assessment |
Retrospective case series |
III |
Foramen magnum osseous abnormalities may contribute to syrinx formation |
| 1 |
Lara-Reyna et al, 202028 |
Patient assessment |
Retrospective case series |
III |
There is variable reduction in syrinx after Chiari decompression in patients with Chiari and syrinx |
| 1 |
Strahle et al, 201529 |
Patient assessment |
Retrospective case series |
III |
Location of CIM syrinx typically have cranial extent in cervical spine, and may be wider than other etiology for syrinx |
| 1 |
Xie et al, 201530 |
Patient assessment |
Retrospective case series |
III |
In patients with CIM and syrinx, PFD may improve syrinx, and patients with improved syrinx may have upward shift of tonsils |
| 2 |
Sadique et al, 202031 |
Diagnostic test |
Prospective/retrospective case series |
III |
There is improvement in peak velocity after decompression, but the authors do not comment on the relationship of changes in velocity to symptoms; it does not answer the question about benefit from decompression as mentioned in the PICO question |
| 2 |
Bapuraj et al, 201632 |
Diagnostic test |
Prospective/retrospective case series |
III |
AMV at aqueduct improves after surgery, but no change in APV. This may correlate with symptom improvement |
| 2 |
McGirt et al, 200533 |
Diagnostic test |
Retrospective case series |
III |
Patients with occipital headaches had obstructed flow and decompression helped those patients but there is no data on whether there is improvement of CSF flow parameters in these patients; only headache improvement is included |
| 2 |
McGirt et al, 200834 |
Diagnostic test |
Prospective/retrospective case series |
III |
Those with ventral and dorsal CSF flow abnormalities showed highest likelihood of symptom improvement/lack of symptom recurrence; does not include postoperative CSF flow data |
| 2 |
McGirt et al, 200615 |
Diagnostic test |
Retrospective case series |
III |
Lack of preoperative CSF obstruction predicts symptom recurrence, but age of each patient is unclear; mean age is 16 ± 13 years, no other details regarding age are provided |
| 2 |
Ventureyra et al, 200335 |
Diagnostic test |
Retrospective case series |
III |
Patients with syrinx, absent flow, and CIM improved after posterior fossa decompression; those with no syrinx may improve with bone only decompression |
| 2 |
Lara-Reyna et al, 202028 |
Diagnostic test |
Prospective/retrospective case series |
III |
Does not consider the value of advanced imaging at predicting benefit from decompression |
| 2 |
Radmanesh et al, 201536 |
Diagnostic test |
Prospective/retrospective case series |
III |
No change in CCOS after surgery even though there was change in the tonsillar pulsatility |
| 2 |
Ellenbogen et al, 200037 |
Diagnostic test |
Prospective/retrospective case series |
III |
All patients had improvement in CSF flow parameters, and pediatric patients mostly improved symptomatically (all except 1) |
| 3 |
Bollo et al, 20128 |
Patient assessment/therapy |
Retrospective case series |
III |
CM 1.5, basilar invagination, CXA <125 degrees are at increased risk of instability and requirement for fusion |
| 3 |
Ravindra et al 202038 |
Diagnostic test |
Prospective case series |
III |
C-C2SVA >5 mm may be predictive of the need for ventral decompression or OC fusion to better understand the anatomic load-bearing relationship between the atlantooccipital joint and the upper cervical spine and its influence on the clinical behavior of patients with CIM and craniocervical pathology |
| 3 |
CreveCoeur et al 202139 |
Diagnostic test/therapy |
Prospective case series |
III |
Platybasia, Klippel–Feil, BV predictive of OCF; BV predictive of OCF/VD; CXA lower in OCF and OCF/VD groups compared with PFD only to examine factors influencing the use of OCF and OCF/VD in a multicenter cohort of pediatric CIM and SM subjects treated with PFD; both retrospective and prospective |
| 3 |
Grabb et al, 199940 |
Diagnostic test |
Retrospective case series |
III |
pb-C2 >9 mm may require stabilization due to neurologic symptoms and instability but very few patients in this study that meet that criterion; 1) to determine the incidence and degree of VBSC in pediatric and young adult patients with CIM and 2) to correlate VBSC with other imaging and clinical factors to help determine what amount of VBSC is successfully treated with a posterior decompressive procedure alone |
AMV, amplitude of mean velocity; APV, amplitude of peak velocity; C-C2SVA, C2 sagittal vertebral alignment; CCOS, Chicago Chiari Outcome Scale; CM, Chiari malformation; CSF, cerebrospinal fluid;
CXA, clivoaxial angle; DX, diagnosis; MRI, magnetic resonance imaging; OC, occipitocervical; OCF, posterior fossa decompression and fusion; TCS, tethered cord syndrome; VBSC, ventral brain stem compression; VD, ventral decompression.
Appendix V. Conflicts of interest
| Name |
Affiliation |
Type of COI |
| Toba Niazi, MD |
Live Like Bella Foundation, Nicklaus Children's Hospital |
Grants/Research Support |
| Laurie Ackerman, MD |
Park Reeves Consortium |
Grants/Research Support |
| David Bauer, MD |
None |
|
| Brandon G. Rocque, MD, MS, FAANS |
None |
|
| Carolyn S. Quinsey, MD |
None |
|
| Eric Jackson, MD |
None |
|
| Jogi V. Pattisapu MD FAAP FAANS |
J&J, Integra |
Consultant |
| Rabia Qaiser, MD |
None |
|
| Cormac O. Maher, MD, FAAP, FACS, FAANS |
None |
|
| Shobhan H. Vachhrajani MD, PhD, FRCSC |
None |
|
| Libby Infinger, MD |
None |
|
| Howard Silberstein, MD |
None |
|
| Sarah Jernigan, MD |
None |
|
| Jeffrey S. Raskin MS MD FAANS FAAP |
None |
|
| Dorothy Poppe |
None |
|
| Kaitlyn Esposito |
None |
|