Guidelines on the Management of Patients with Vestibular Schwannoma
3. Hearing Preservation Outcomes in Patients with Sporadic Vestibular Schwannomas
download pdf Neurosurgery, 2017
Sponsored by: Congress of Neurological Surgeons (CNS) and the AANS/CNS Tumor Section
Endorsed by: Joint Guidelines Committee of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS)
Authors:
Matthew L. Carlson, MD1,2, Esther X. Vivas, MD3, D. Jay McCracken, MD4, Alex D. Sweeney, MD5, Brian A. Neff, MD1,2, Neil T. Shepard, PhD1, Jeffrey J. Olson, MD4
1Department of Otorhinolaryngology, Mayo Clinic, School of Medicine, Rochester, Minnesota, USA
2Department of Neurologic Surgery, Mayo Clinic, School of Medicine, Rochester, Minnesota, USA
3Department of Otolaryngology-Head & Neck Surgery, Emory University School of Medicine, Atlanta, Georgia, USA
4Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia, USA
5Bobby R. Alford Department of Otolaryngology-Head and Neck Surgery, Baylor College of Medicine, Houston, Texas, USA
Correspondence:
Matthew L. Carlson, MD
Department of Otorhinolaryngology
Department of Neurosurgery
Mayo Clinic, 200 First St. SW
Rochester, Minnesota 55905
Telephone: 507-284-8532
Fax: 507-284-3907
Email: carlson.matthew@mayo.edu
Keywords: Acoustic neuroma, hearing loss, hearing preservation, radiosurgery, skull base, surgery, vestibular schwannoma
Abbreviations
AAO-HNS: American Academy of Otolaryngology-Head and Neck Surgery
CSF: Cerebrospinal fluid
GR: Gardner–Robertson hearing classification
HL: Hearing loss
NF2: Neurofibromatosis type 2
VS: Vestibular schwannoma
No part of this manuscript has been published or submitted for publication elsewhere.
Abstract
Radiation
Question
What is the overall probability of maintaining serviceable hearing following single-fraction radiation therapy, utilizing modern dose planning, at two years, five years, and ten years following treatment?
Target population
These recommendations apply to all adults with sporadic vestibular schwannomas who have documented serviceable hearing in the ipsilateral ear prior to treatment and have received single-fraction stereotactic radiation, using ≤ 13 Gy to the tumor margin.
Recommendation
Level 3: Individuals who meet these criteria and are considering stereotactic radiosurgery should be counseled that there is moderately high probability (> 50% to 75%) of hearing preservation at two years, moderately high probability (> 50% to 75%) of hearing preservation at five years, and moderately low probability (> 25% to 50%) of hearing preservation at ten years.
Question
Among patients with AAO-HNS class A or GR grade I hearing at baseline, what is the overall probability of maintaining serviceable hearing following single-fraction radiation therapy, utilizing modern dose planning, at two years, five years, and ten years following treatment?
Target population
These recommendations apply to adults with sporadic vestibular schwannomas who have documented AAO-HNS class A or GR grade I hearing in the ipsilateral ear prior to treatment and have received single-fraction stereotactic radiation using ≤ 13 Gy to the tumor margin.
Recommendation
Level 3: Individuals who meet these criteria and are considering stereotactic radiosurgery should be counseled that there is a high probability (> 75% to 100%) of hearing preservation at two years, moderately high probability (> 50% to 75%) of hearing preservation at five years, and moderately low probability (> 25% to 50%) of hearing preservation at ten years.
Question
What patient- and tumor-related factors influence progression to non-serviceable hearing following single-fraction stereotactic radiation treatment using ≤ 13 Gy to the tumor margin?
Target population
These recommendations apply to adults with sporadic vestibular schwannomas who have serviceable hearing in the ipsilateral ear prior to treatment and have received single-fraction stereotactic radiation using ≤ 13 Gy to the tumor margin.
Recommendation
Level 3: Individuals who meet these criteria and are considering stereotactic radiosurgery should be counseled regarding the probability of successful hearing preservation based on the following prognostic data: the most consistent prognostic features associated with maintenance of serviceable hearing are good preoperative word recognition and/or pure tone thresholds with variable cut-points reported, smaller tumor size, marginal tumor dose ≤ 12 Gy, and cochlear dose ≤ 4 Gy. Age and sex are not strong predictors of hearing preservation outcome.
Surgery
Question
What is the overall probability of maintaining serviceable hearing following microsurgical resection of small to medium-sized sporadic vestibular schwannomas early after surgery, at two years, at five years, and at ten years following treatment?
Target population
These recommendations apply to adults with small to medium-sized (< 2 cm) sporadic vestibular schwannomas who have documented serviceable hearing in the ipsilateral ear prior to microsurgical resection via the middle cranial fossa or retrosigmoid approach.
Recommendation
Level 3: Individuals who meet these criteria and are considering microsurgical resection should be counseled that there is a moderately low probability (> 25% to 50%) of hearing preservation immediately following surgery, moderately low probability (> 25% to 50%) of hearing preservation at two years, moderately low probability (> 25% to 50%) of hearing preservation at five years, and moderately low probability (> 25% to 50%) of hearing preservation at ten years.
Question
Among patients with AAO-HNS class A or GR grade I hearing at baseline, what is the overall probability of maintaining serviceable hearing following microsurgical resection of small to medium-sized sporadic vestibular schwannomas early after surgery, at two years, at five years, and at ten years following treatment?
Target population
These recommendations apply to adults with small to medium-sized (< 2 cm) sporadic vestibular schwannomas who have documented AAO-HNS class A or GR grade I hearing in the ipsilateral ear prior to microsurgical resection via the middle cranial fossa or retrosigmoid approach.
Recommendation
Level 3: Individuals who meet these criteria and are considering microsurgical resection should be counseled that there is a moderately high probability (> 50% to 75%) of hearing preservation immediately following surgery, moderately high probability (> 50% to 75%) of hearing preservation at two years, moderately high probability (> 50% to 75%) of hearing preservation at five years, and moderately low probability (> 25% to 50%) of hearing preservation at ten years.
Question
What patient- and tumor-related factors influence progression to non-serviceable hearing following microsurgical resection of small to medium-sized sporadic vestibular schwannomas?
Target population
These recommendations apply to adults with small to medium-sized (< 2 cm) sporadic vestibular schwannomas who have documented serviceable hearing in the ipsilateral ear prior to microsurgical resection via the middle cranial fossa or retrosigmoid approach.
Recommendation
Level 3: Individuals who meet these criteria and are considering microsurgical resection should be counseled regarding the probability of successful hearing preservation based on the following prognostic data: the most consistent prognostic features associated with maintenance of serviceable hearing are good preoperative word recognition and/or pure tone thresholds with variable cut-points reported, smaller tumor size, commonly < 1 cm, and presence of a distal internal auditory canal cerebrospinal fluid fundal cap. Age and sex are not strong predictors of hearing preservation outcome.
Observation
Question
What is the overall probability of maintaining serviceable hearing with conservative observation of vestibular schwannomas at two years, five years, and ten years following diagnosis?
Target population
These recommendations apply to adults with small to medium-sized sporadic vestibular schwannomas who have documented serviceable hearing in the ipsilateral ear at time of diagnosis.
Recommendation
Level 3: Individuals who meet these criteria and are considering observation should be counseled that there is a high probability (> 75% to 100%) of hearing preservation at two years, moderately high probability (> 50% to 75%) of hearing preservation at five years, and moderately low probability (> 25% to 50%) of hearing preservation at ten years.
Question
Among patients with AAO-HNS class A or GR grade I hearing at baseline, what is the overall probability of maintaining serviceable hearing with conservative observation at two years, and five years following diagnosis?
Target population
These recommendations apply to adults with small to medium-sized (< 2 cm) sporadic vestibular schwannomas who have documented class A or GR grade I hearing in the ipsilateral ear at time of diagnosis.
Recommendation
Level 3: Individuals who meet these criteria and are considering stereotactic radiosurgery should be counseled that there is a high probability (> 75% to 100%) of hearing preservation at two years, and moderately high probability (> 50% to 75%) of hearing preservation at five years. Insufficient data were available to determine the probability of hearing preservation at ten years for this population subset.
Question
What patient- and tumor-related factors influence progression to non-serviceable hearing during conservative observation?
Target population
These recommendations apply to adults with small to medium-sized (< 2 cm) sporadic vestibular schwannomas who have documented serviceable hearing in the ipsilateral ear at time of diagnosis.
Recommendation
Level 3: Individuals who meet these criteria and are considering observation should be counseled regarding probability of successful hearing preservation based on the following prognostic data: the most consistent prognostic features associated with maintenance of serviceable hearing are good preoperative word recognition and/or pure tone thresholds with variable cut-points reported, as well as non-growth of the tumor. Tumor size at the time of diagnosis, age, and sex do not predict future development of non-serviceable hearing during observation.
Introduction
Rationale
Over the last 100 years, there has been a significant shift in VS outcome priorities.1,2 Prior to Harvey Cushing’s monumental treatise in 1917, Tumors of the Nervus Acusticus and Syndrome of the Cerebellopontile Angle, the mortality of surgery for VSs reached 80%.3 Early advances pioneered by Cushing, and later his protégé and rival Walter Dandy, resulted in an unprecedented ~50% reduction in mortality at a time when tumors commonly presented late in course with hydrocephalus.1-4 However, despite such improvements, permanent cranial nerve injury was common and considered an unavoidable compromise for the treatment of life-threatening tumor growth.
Advancements in technology and surgical techniques during the 1950s and 1960s culminated in the application of the surgical microscope and electrical dental drill to VS surgery by William House.5-8 In addition, it was during this time that the subtemporal middle cranial fossa and translabyrinthine approaches were revitalized after being abandoned nearly 60 years earlier because of technical prematurity.5,7,9,10 Simultaneously, Lars Leksell, a pupil of the preeminent neurosurgeon Herbert Olivecrona of Sweden, pioneered the development of his arc centered stereotactic frame as a means of noninvasive, precise ablation of intracranial lesions utilizing convergent beam radiation.11,12 In reaction to witnessing the morbidity of surgical resection even in the best hands, in 1971, Leksell published the inaugural account of VS treatment using stereotactic radiation.13 These simultaneous advancements in microsurgery and radiosurgery ushered the transition of priority from life preservation to cranial nerve preservation.2 For the first time in the history of VS management, tumors could be effectively treated with the intent of tumor control and facial nerve preservation. Successive advances in technique and neuromonitoring facilitated further improvements in facial nerve outcomes and hearing preservation via the middle cranial fossa and transmeatal retrosigmoid craniotomy.14,15
The most recent era in VS treatment was enabled by developments in noninvasive neuroimaging, including contrast-enhanced computed tomography and magnetic resonance imaging. In this setting, tumor observation with serial imaging became a viable strategy. Initially, only patients with minimal symptoms, small tumors, advanced age, or severe comorbidities were considered for a conservative “wait-and-scan” strategy; however, over time, this approach has been adopted with increasing frequency.16,17 Since 1976, Gentofte University Hospital of Copenhagen Denmark has pioneered the reporting of VS natural history data, where a national centralized care center for VS treatment has been maintained.18,19
The evolution in treatment over the last century has ultimately led to an environment where functional outcome has taken precedence over disease eradication.10 With multiple noninvasive management options available, the tolerance of cranial neuropathy in patients with small to medium-sized tumors is low. Today, hearing preservation, facial nerve function, and tumor control remain the primary benchmarks used to evaluate treatment effectiveness and compare outcomes.
Unilateral hearing loss (HL) is associated with impairment in speech understanding in noise and sound localization, leading to a reduction in quality of life.20-22 In addition, binaural hearing remains critical to occupation performance for some, including individuals involved in law enforcement or military service, for example. Furthermore, progressive HL from a VS in an only hearing ear can be functionally devastating.22 Thus, characterizing HL over time following treatment or conservative observation is critical, particularly in the setting of “benign” disease where patients are expected to live many decades beyond diagnosis and the treatment and effects of age-related HL will only compound hearing disability from disease.
Unfortunately, data in the VS literature regarding long-term hearing preservation are conflicting. Fueled by disparate study methodology and heterogeneous reporting, a general consensus regarding realistic expectations of long-term preservation of useful hearing is lacking.23-25 For example, there are at least 8 different hearing classification systems that have been used in the literature, and in many reports, “hearing preservation” simply refers to maintenance of any detectable hearing, regardless of functionality.26-35 Even when hearing preservation rates are reported, it is not always clear what percentage of patients started with useful hearing, which of course is critical to understand when comparing between studies and comparing treatment modalities.25 Within these classification systems, the cutoff for “useful” or “serviceable” hearing is often different. In addition, study inclusion and treatment selection bias often limits the clinician’s ability to draw strong conclusions that can be applied to the general VS population.
Objectives
This systematic review and clinical practice guideline focuses on summarizing the probability of hearing preservation within the first 10 years after contemporary stereotactic radiation delivery, microsurgery, or observation with serial imaging. In addition, candidate prognostic features, such as tumor size and location, patient age, pretreatment hearing status, and others are explored.
Notably, this systematic review and clinical practice guideline concentrates primarily on patient- and tumor-related factors. Detailed analysis of radiosurgical planning parameters, cochlear shielding strategies, comparison of surgical approaches, and methods of eighth nerve monitoring are deferred because they are reviewed thoroughly in other guidelines in this series.
Methods
Process Overview
The evidence-based clinical practice guideline task force members and the Joint Tumor Section of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS) conducted a systematic review of the literature relevant to the management of VSs. Additional details of the systematic review are provided below and within the introduction and methodology chapter of the guideline (here).
Article Inclusion/Exclusion Criteria
One thousand three hundred and seven citations were manually reviewed by the team with specific inclusion and exclusion criteria as outlined below. Three independent reviewers reviewed and abstracted full-text data for each article, and the 2 sets of data were compared for agreement by a third party. Inconsistencies were re-reviewed, and disagreements were resolved by consensus. To be included in this guideline, an article has to be a study that:
General
- Investigated patients suspected of having VSs
- Was of humans
- Was not an in vitro study
- Was not a biomechanical study
- Was not performed on cadavers
- Was published between January 1, 1990 and December 31, 2014
- Was published in a peer-reviewed journal
- Was not a meeting abstract, editorial, letter, or a commentary
- Was published in English
- Included quantitatively presented results
Specific
- Used the 1995 AAO-HNS26 or GR hearing classification system29 or presented data using a cut-off of ≥50% word recognition score and ≤50 dB pure tone average for defining serviceable hearing or had individual patient data presented such that the latter criteria could be applied and analyzed
- For patients receiving single fraction radiation therapy, a contemporary dose plan using ≤13 Gy to the tumor margin36,37
- Included a median or mean follow-up of at least 2 years following treatment
- Included a minimum of 20 patients
- Studies focusing on NF2 or those reporting outcomes in sporadic and NF2-associated tumors, without providing separate outcome data, were not included for review
The authors did not include systematic reviews, guidelines, or meta-analyses conducted by other authors. These documents were developed using different inclusion criteria than those specified in this guideline. Therefore, they may have included studies that do not meet the inclusion criteria stated above. The authors recalled these documents if their abstracts suggested that they might address one of the recommendations presented here, and the bibliographies were searched for additional studies.
Search Strategies
The task force collaborated with a medical librarian to search for articles published between January 1, 1990 and December 31, 2014. Three electronic databases were searched: PubMed, EMBASE, and Web of Science. Strategies for searching electronic databases were constructed by the evidence-based clinical practice guideline task force members and the medical librarian using previously published search strategies to identify relevant studies (Table 1; Figure 1).38-45
The authors supplemented searches of electronic databases with manual screening of the bibliographies of all retrieved publications. The authors also searched the bibliographies of recent systematic reviews and other review articles for potentially relevant citations. All articles identified were subject to the study selection criteria listed above. As noted above, the guideline committee also examined lists of included and excluded studies for errors and omissions. The authors went to great lengths to obtain a complete set of relevant articles. Having a complete set ensures that the guideline is not based on a biased subset of articles.
Data Analysis
Evidence tables for radiation treatment, microsurgery, and observation were constructed using key study parameters as outlined above. In addition, the percentage of patients who maintained useful hearing at time points between 1 and 10 years and who had serviceable hearing at baseline was recorded according to data available in each study. “Serviceable hearing” or “useful hearing” was defined by a word recognition score of ≥50% and a pure tone average or speech response threshold of ≤50 dB HL, which is equivalent to AAO-HNS class A-B and GR grade I-II.26,29 The aggregate data obtained from individual studies were summarized via a weighted average to determine the overall percentage of patients with useful hearing at years 1 through 10 for each treatment modality. To accommodate a range of outcomes between studies, 4 ordinal categories of probability were devised for the purpose of guideline formulation: “high probability” of hearing preservation defined by >75% to 100%, “moderately high probability” defined by >50% to 75%, “moderately low probability” defined by >25% to 50%, and “low probability” defined by 0% to 25%.
Classification of Evidence and Guideline Formulation
The concept of linking evidence to recommendations has been further formalized by the American Medical Association and many specialty societies, including the AANS, the CNS, and the American Academy of Neurology. This formalization involves the designation of specific relationships between the strength of evidence and the strength of recommendations to avoid ambiguity. In the paradigm for prognostication used in this guideline, evidence is classified into 1 of 3 tiers based upon the degree at which the study fulfills 5 technical criteria as outlined below:
- Was a well-defined representative sample of patients assembled at a common (usually early) point in the course of their disease?
- Was patient follow-up sufficiently long and complete?
- Were objective outcome criteria applied in a “blinded” fashion?
- If subgroups with different prognoses were identified, was there adjustment for important prognostic factors?
- If specific prognostic factors were identified, was there validation in an independent “test set” group of patients?
Class I evidence is used to support recommendations of the strongest type, defined as Level 1 recommendations, and require that all 5 technical criteria are satisfied. Class II evidence supports intermediate strength recommendations, defined as level 2 recommendations, and require that 4 of the 5 technical criteria be met. Finally, Class III evidence supports level 3 recommendations, comprising all remaining studies that satisfy 3 or less of the 5 technical criteria. A basis for these guidelines can be viewed in Haines SJ and Nicholas JS (2006). Evidence-Based Medicine: A Conceptual Framework. In Haines SJ and Walters BC (Eds.), Evidence-Based Neurosurgery: An Introduction (Pages 1-17). New York: Thieme Medical Publishers.
Results
Radiation
Question 1
What is the overall probability of maintaining serviceable hearing following single-fraction radiation therapy, utilizing modern dose planning, at 2 years, 5 years, and 10 years following treatment?
Target population
These recommendations apply to all adults with sporadic vestibular schwannomas who have documented serviceable hearing in the ipsilateral ear prior to treatment and have received single-fraction stereotactic radiation using ≤13 Gy to the tumor margin.
Recommendation
Level 3: Individuals who meet these criteria and are considering stereotactic radiosurgery should be counseled that there is moderately high probability (>50% to 75%) of hearing preservation at 2 years, moderately high probability (>50% to 75%) of hearing preservation at 5five years, and moderately low probability (>25% to 50%) of hearing preservation at 10 years.
Question 2
Among patients with AAO-HNS class A or GR grade I hearing at baseline, what is the overall probability of maintaining serviceable hearing following single-fraction radiation therapy, utilizing modern dose planning, at 2 years, 5 years, and 10 years following treatment?
Target population
These recommendations apply to adults with sporadic vestibular schwannomas who have documented AAO-HNS class A or GR grade I hearing in the ipsilateral ear prior to treatment and have received single-fraction stereotactic radiation using ≤13 Gy to the tumor margin.
Recommendation
Level 3: Individuals who meet these criteria and are considering stereotactic radiosurgery should be counseled that there is a high probability (>75% to 100%) of hearing preservation at 2 years, moderately high probability (>50% to 75%) of hearing preservation at 5 years, and moderately low probability (>25% to 50%) of hearing preservation at 10 years.
Question 3
What patient- and tumor- related factors influence progression to nonserviceable hearing following single-fraction stereotactic radiation treatment using ≤13 Gy to the tumor margin?
Target population
These recommendations apply to adults with sporadic vestibular schwannomas who have serviceable hearing in the ipsilateral ear prior to treatment and have received single-fraction stereotactic radiation using ≤13 Gy to the tumor margin.
Recommendation
Level 3: Individuals who meet these criteria and are considering stereotactic radiosurgery should be counseled regarding the probability of successful hearing preservation based on the following prognostic data: the most consistent prognostic features associated with maintenance of serviceable hearing are good preoperative word recognition and/or pure tone thresholds with variable cut-points reported, smaller tumor size, marginal tumor dose ≤12 Gy, and cochlear dose ≤4 Gy. Age and sex are not strong predictors of hearing preservation outcome.
Study Selection
A total of 1307 studies were screened and assessed for eligibility, and 47 publications were included in the final review.46-92 Specific to these recommendations, only studies evaluating single-fraction stereotactic radiation therapy using modern treatment paradigms, including a median dose of ≤13 Gy to the tumor margin, with a minimum of 20 patients, and a median or mean of at least 2 years of follow-up are included. As a separate additional analysis, studies incorporating fractionated treatment strategies were also summarized (See Additional Analysis below).
Study Characteristics
Data extraction included study design, class of evidence, primary treatment modality, total number of patients, number of patients with pretreatment serviceable hearing, study selection parameters, mean or median tumor size, mean or median follow-up, inclusion of NF2, inclusion of recurrent VSs, percentage of patients with serviceable hearing between 1 and 10 years, and prognostic features associated with the development of nonserviceable hearing.
Risk of Bias and Study Limitations
All selected publications were retrospective or nonrandomized prospective studies, and therefore there is substantial risk of treatment selection bias. For example, some centers may be more likely to observe small tumors in patients with good hearing, while others may consider upfront radiosurgery or microsurgery with an attempt at hearing preservation.93-97 Patients with tumors larger than 1.5 to 2 cm in maximum posterior fossa dimension are not commonly considered candidates for hearing preservation surgery given the low probability of success, even when good preoperative hearing is present; however, such patients are generally included in radiosurgical series reporting hearing preservation outcomes.98-100 In addition, because most studies only include a single treatment arm, our ability to isolate the effect of radiation on HL from the natural history of progressive decline inherent to having a VS is difficult. Finally, an attempt to control for variance in radiation planning parameters was made by limiting inclusion to only those publications primarily using a lower (≤13 Gy) marginal dose.36,37 Because of the tremendous heterogeneity in fractionation schedules and dosing, studies analyzing the results of fractionated radiation therapy were not included in the primary analysis, but are reported separately.
Results of Individual Studies
The key results of individual studies are outlined in Table 2 and are summarized within the guideline recommendations. There were 4 publications that met study criteria and included both a radiation cohort and an observation control arm.62,65,69,92 These publications offer a special opportunity to examine the effects of radiation on HL over the natural history of audiometric decline and are discussed in this section. In addition, there are 2 studies with Class II evidence comparing radiosurgery and microsurgery; however, these studies will be specifically addressed in the final discussion when all 3 treatment modalities are collectively reviewed.71,75
In 2010, Regis et al69 presented a consecutive series of 47 patients with intracanalicular VSs who were managed with conservative observation and 34 patients with intracanalicular tumors who received proactive radiosurgery using a median dose of 12 Gy to the tumor margin. They found that of the 31 patients with serviceable hearing at the time of observation commencement, 21 (68%) maintained useful hearing. When comparing the observation and radiosurgery groups using Kaplan–Meier analysis at 3, 4, and 5 years, 75%, 52%, and 41% of patients in the observation cohort maintained serviceable hearing, respectively. This is compared to 77%, 70%, and 64% at the same time points for the cohort receiving upfront radiosurgery. The authors concluded that proactive radiosurgery conferred a greater chance of hearing preservation than observation. However, there are no statistical comparisons performed between groups that strictly evaluated hearing preservation. In addition, in this study, the rate of tumor growth in the observation group was over 4 times greater than was reported by other large studies, with 77% demonstrating growth in just over 3 years. Furthermore, the authors do not explicitly define tumor enlargement, other than “significant tumor growth.”
In 2012, Rasmussen et al62 compared the outcomes of 42 patients who received fractionated radiation therapy to a historical cohort of 409 control subjects who received observation and were matched by initial hearing levels. They reported that at 2 years after radiation therapy, only 8 of an initial 21 (38%) patients with serviceable hearing maintained GR grade I or II hearing, and at 10 years all had progressed to nonserviceable hearing. This is compared to 60% who maintained GR grade 1 hearing in the observation cohort. In addition, in contrast to Regis et al,69 only 12% demonstrated growth (>2 mm) during trial observation. Notably, however, in the study by Rasmussen et al,62 patients were only treated with radiation after tumor growth was detected rather than receiving proactive treatment as reported by Regis et al69
In 2013, Breivik et al92 prospectively compared an observational cohort (n = 124) to a radiosurgical arm (n = 113) receiving 12 Gy to the margin, and all 237 patients had tumors with extracanalicular extension. At a mean follow-up of 55 months, 17 of 71 (24%) conservatively managed patients with serviceable hearing at baseline maintained GR grade I or II hearing, compared to 19 of 53 (36%) who received radiosurgery. It is notable that treatment was not randomized, but followed an institutional algorithm. Based on this, the radiosurgery group contained larger tumors at baseline, but otherwise there were no other important differences between groups prior to treatment. The authors concluded that radiosurgery does not appear protective, nor does it appear to accelerate HL compared to observation. It is critical to note that Regis et al69 only included intracanalicular tumors, while Breivik et al92 only analyzed tumors with extracanalicular extension; the results of these 2 studies are therefore not freely comparable.
In the remaining study, Kim et al65 evaluated a cohort of 41 patients with serviceable pretreatment hearing who underwent radiosurgery and compared this to a historical cohort of 15 patients who were managed with observation. However, analyses comparing the radiosurgery and observation cohorts were only made for 19 of the radiosurgery patients who experienced acute hearing decline and received glucocorticoid therapy. For these reasons, the latter comparative study is not discussed further in this section.
Synthesis of Results
Class III evidence supports the conclusion that the risk of HL increases with time, well beyond the first 2 years following radiation treatment. When evaluating all patients with serviceable hearing at baseline, approximately 72% will maintain serviceable hearing at 2 years, 63% at 5 years, and 33% at 10 years. Currently, there are 2 studies with Class II evidence comparing audiometric decline following radiosurgery to conservative management: 1 suggesting a protective effect of radiation, and 1 supporting no significant difference between groups.69,92
Additional Analysis
The collective results of fractionated radiation therapy for sporadic VSs were separately analyzed. A total of 16 studies met study inclusion criteria and were analyzed.46,47,49,50,53,55,57,62,64,67,73,80,82,83,91,101 Of these, 1 study compared fractionated radiation to conservative observation. Lin et al101 reported the results of 16 patients who received hyperfractionated radiation therapy, 113 who underwent microsurgery, and 86 who were initially managed with conservative observation. However, only 11 patients within the radiation arm had serviceable hearing at baseline. For these reasons, the latter comparative study is not discussed further in this section. Overall, the probability of maintaining serviceable hearing after contemporary fractionated radiation therapy was 85% at 2 years and 72% at 5 years; however, there was tremendous heterogeneity in the treatment parameters and a wide range of outcomes between studies, making it impossible to draw any definitive conclusions regarding this subgroup.
Discussion
In reviewing the literature, there has been 1 recent large review in the VS literature evaluating hearing preservation following radiation therapy. In 2010, Yang et al102 identified 45 articles in the literature, which summarized 4234 patients. They found that overall, 51% of patients with serviceable hearing at baseline maintained useful hearing at a mean of 44 months following radiation. However, when only including those who received a dose of ≤13 Gy to the margin, 60.5% maintained serviceable hearing. This is within 3% of the current study estimate for the 4-year time point. They found that size and age did not predict future development of nonserviceable hearing; however, tumor dose to the margin was strongly associated with HL. Yang et al102 did not provide time point estimates of hearing preservation in their study.
Summary
The evidence for this guideline was primarily drawn from studies with Class III evidence and a limited number with class II evidence; currently, no class I evidence exists to guide recommendations on this topic. These data should be used when counseling patients regarding the probability of long-term maintenance of serviceable hearing following contemporary low-dose radiation therapy for sporadic VSs. The risk of developing nonserviceable hearing is cumulative over time, and at 10 years, less than half of patients who begin with serviceable hearing will maintain useful hearing levels.
Surgery
Question 4
What is the overall probability of maintaining serviceable hearing following microsurgical resection of small to medium-sized sporadic vestibular schwannomas early after surgery, at 2 years, at 5 years, and at 10 years following treatment?
Target population
These recommendations apply to adults with small to medium-sized (<2 cm) sporadic vestibular schwannomas who have documented serviceable hearing in the ipsilateral ear prior to microsurgical resection via the middle cranial fossa or retrosigmoid approach.
Recommendation
Level 3: Individuals who meet these criteria and are considering microsurgical resection should be counseled that there is a moderately low probability (>25% to 50%) of hearing preservation immediately following surgery, moderately low probability (>25% to 50%) of hearing preservation at 2 years, moderately low probability (>25% to 50%) of hearing preservation at 5 years, and moderately low probability (>25% to 50%) of hearing preservation at 10 years.
Question 5
Among patients with AAO-HNS class A or GR grade I hearing at baseline, what is the overall probability of maintaining serviceable hearing following microsurgical resection of small to medium-sized sporadic vestibular schwannomas early after surgery, at 2 years, at 5 years, and at 10 years following treatment?
Target population
These recommendations apply to adults with small to medium-sized (<2 cm) sporadic vestibular schwannomas who have documented AAO-HNS class A or GR grade I hearing in the ipsilateral ear prior to microsurgical resection via the middle cranial fossa or retrosigmoid approach.
Recommendation
Level 3: Individuals who meet these criteria and are considering microsurgical resection should be counseled that there is a moderately high probability (>50% to 75%) of hearing preservation immediately following surgery, moderately high probability (>50% to 75%) of hearing preservation at 2 years, moderately high probability (>50% to 75%) of hearing preservation at 5 years, and moderately low probability (>25% to 50%) of hearing preservation at 10 years.
Question 6
What patient- and tumor-related factors influence progression to nonserviceable hearing following microsurgical resection of small to medium-sized sporadic vestibular schwannomas?
Target population
These recommendations apply to adults with small to medium-sized (<2 cm) sporadic vestibular schwannomas who have documented serviceable hearing in the ipsilateral ear prior to microsurgical resection via the middle cranial fossa or retrosigmoid approach.
Recommendation
Level 3: Individuals who meet these criteria and are considering microsurgical resection should be counseled regarding the probability of successful hearing preservation based on the following prognostic data: the most consistent prognostic features associated with maintenance of serviceable hearing are good preoperative word recognition and/or pure tone thresholds with variable cut-points reported, smaller tumor size commonly less than 1 cm, and presence of a distal internal auditory canal cerebrospinal fluid fundal cap. Age and sex are not strong predictors of hearing preservation outcome.
Study Selection
A total of 1307 studies were screened and assessed for eligibility, and 37 were included in the final review.71,75,103-136 Specific to this recommendation, only studies evaluating outcomes with the intent of hearing preservation using the middle cranial fossa or retrosigmoid/suboccipital craniotomy, with a minimum of 20 patients, and with a median or mean of at least 2 years of follow-up are included.
Study Characteristics
Data extraction included study design, class of evidence, primary treatment modality, total number of patients, number of patients with pretreatment serviceable hearing, study selection parameters, mean or median tumor size, mean or median follow-up, inclusion of NF2, inclusion of recurrent VSs, percentage of patients with serviceable hearing between 1 and 10 years, and prognostic features associated with the development of nonserviceable hearing.
Risk of Bias and Study Limitations
Because all selected publications were either retrospective or nonrandomized prospective studies, there is a substantial risk of treatment selection bias. Specific to microsurgery for hearing preservation, commonly only ideal candidates, including those with good existing hearing and small tumor size, are considered for hearing preservation. In addition, because most studies only include a single treatment arm, it is difficult to isolate the contribution of surgery to immediate and delayed deterioration of hearing decline from the natural history of progressive decline inherent to having a VS. Finally, hearing preservation outcome analysis is particularly problematic for retrosigmoid craniotomy, because the intent of hearing preservation is not always adequately outlined in the study. Specifically, some surgeons prefer the retrosigmoid approach even in cases where hearing preservation is not attempted, such as for medium- or large-sized tumors.10 Tumor selection by approach also comes into play when comparing retrosigmoid and middle fossa craniotomy. That is, medial tumors with greater cerebellopontine angle extension are more commonly managed with the retrosigmoid approach, whereas smaller lateral based tumors are more frequently selected for the middle fossa approach. Therefore, when comparing outcomes, it is critical that the same size class is compared because size is one of the primary predictors of hearing preservation outcome.
Results of Individual Studies
The key results of individual studies are outlined in Table 3, and are summarized within the guideline recommendations. There were 2 publications that met study criteria and included a microsurgical cohort and an observation control arm.101,115 These 2 publications offer a special opportunity to examine the effects of surgery on HL over the natural history of audiometric decline and are discussed in this section. In addition, there are 2 studies with class II evidence comparing radiosurgery and microsurgery; however, these studies will be specifically addressed in the final discussion when all 3 treatment modalities are reviewed.71,75
In 2005, Grayeli et al115 compared the results of microsurgery and conservative observation in a cohort of 416 unilateral VSs: 114 intracanalicular and 302 with ≤15 mm in greatest cisternal dimension. The 111 conservatively managed patients consisted of those over 60 years of age and those who had contraindications or refused surgery. The mean follow-up was 33 months, and 47% demonstrated radiological growth of at least 2 mm. Of the 44 patients who presented with serviceable hearing, 25 (57%) maintained AAO-HNS class A or B at last follow-up. The mean follow-up in the microsurgery arm was 18 months. Initially, 183 patients had serviceable hearing at baseline and of these, 145 underwent attempted hearing preservation via the middle fossa or retrosigmoid approach. Of the latter, 45 (31%) maintained serviceable hearing at one year following surgery. Longer follow-up in both groups would have been beneficial to determine if serviceable hearing following surgery was durable, and to determine the rate of continued decline in the observation cohort.
In 2005, Lin et al101 published a retrospective study comparing hearing preservation outcomes consisting of a group of 16 patients who received hyperfractionated radiation therapy (50 Gy, 25 fractions over 5 weeks), 113 patients who received retrosigmoid craniotomy for hearing preservation microsurgery, and 51 patients who were managed with conservative observation. With the microsurgical arm, 30 (27%) had serviceable hearing in the immediate postoperative period, and over a mean follow-up of 9.5 years, 18 (16%) maintained long-term useful hearing. Of the patients managed with conservative observation, 22 of 51 (43%) maintained GR grade I-II hearing at a mean follow-up of 6.8 years. Finally, only 1 of 11 (9%) patients who received radiation therapy maintained serviceable hearing at a mean follow-up of 4 years. In this study, the rate of initial hearing preservation following microsurgery for tumors <2 cm was relatively low; however, it is notable that only 10% of patients progressed to nonserviceable hearing after a follow-up of nearly 10 years if useful hearing was initially preserved. This is in contrast to the higher percentage of decline that occurred in the radiation and observation cohorts over shorter durations of follow-up.
To further highlight the difference in the pattern of HL after microsurgery compared to radiation therapy and observation, 4 additional studies reporting long-term follow-up are summarized here. In 2003, Chee, Nedzelski, and Rowed119 found that among patients who had serviceable hearing immediately following retrosigmoid tumor resection, 15 of 23 (65%) patients maintained useful hearing at a mean follow-up of 9.5 years following surgery. In 2010, Sughrue et al137 evaluated surgical outcomes in patients less than 40 years of age and found that if hearing was initially preserved, no patients progressed to nonserviceable hearing in the operated ear even after 10 years of follow-up. In 2014, Quist et al138 reported that 12 of 16 (75%) patients who had hearing initially preserved following middle fossa tumor resection maintained AAO-HNS class A or B hearing after 5 years of follow-up. As a limitation, 11 additional patients did not have long-term audiometric data available and were excluded from the final analysis. In 2014, Yamakami et al103 reported that 80% (12/15) of patients who initially had hearing preserved following microsurgery maintained useful hearing at a median follow-up of 7 years. Similarly, 11 patients did not have long-term audiometric data reported. Thus, collectively, these data demonstrate that if hearing can be successfully preserved immediately following surgery, 65-100% of patients maintain durable useful hearing long term.
Synthesis of Results
Class III evidence supports the conclusion that the greatest risk to hearing with surgery occurs upfront. If hearing is initially preserved following surgery, the results tend to be durable. This is in contrast to conservative observation and radiation where the immediate risk is low, but delayed or protracted loss of serviceable hearing is common.58,139 When evaluating all patients with small to medium-sized (<2 cm) sporadic VS with serviceable hearing prior to surgery, and including patients who lost useful hearing immediately following surgery, 47% will maintain serviceable hearing at 2 years, 45% at 5 years, and 43% at 10 years.
Discussion
In searching the literature, there have been several recent large reviews evaluating hearing preservation following microsurgical resection. In 2010, Sughrue et al140 reported on the 998 patients from 49 articles who met inclusion criteria. Only patients with serviceable preoperative hearing were included and an attempt to remove duplicate patient accounts was made. Overall, 286 patients underwent middle fossa craniotomy, and 702 patients underwent the retrosigmoid approach. The percentage of patients with hearing preservation was 52% over a follow-up of 6 months to 7 years. On univariate analysis, the authors found that age greater than 60 years, increasing tumor size, retrosigmoid approach, and gross total removal (vs. subtotal removal) were associated with a greater risk of loss of serviceable hearing. On multivariate analysis, a retrosigmoid approach (odds ratio = 4.2 [95% confidence interval = 2.0–8.8]; P < .001) and size >1.5 cm (odds ratio = 2.8 [95% confidence interval = 1.6–5.0], P < .001) were the only factors that remained statistically significant to predict loss of serviceable hearing. Unfortunately, data regarding change in hearing over follow-up was not described.
In 2012, Ansari et al141 published a literature review evaluating 5064 patients from 35 studies. Inclusion criteria mandated that studies reported pre- and postoperative data using the AAO-HNS criteria (or its equivalent).141 However, “HL” included patients with less than AAO-HNS class B hearing, a pure-tone average of greater than 50 dB HL, or a speech discrimination score of less than 50%. When comparing outcomes between categorical tumor size groups of <1.5 cm, 1.5-3.0 cm, and >3 cm, the middle fossa approach demonstrated a 64% hearing preservation rate for tumors <1.5 cm, compared to 44% for retrosigmoid craniotomy (P < .001). This study also demonstrated that facial nerve outcomes were superior for intracanalicular tumors using the retrosigmoid approach. The results of these studies are not contradictory with the findings of the current systematic review. However, because many of the aforementioned reviews do not report HL at individual time points, the results of these studies cannot be directly compared to the current systematic review.
Summary
The evidence for this guideline was primarily drawn from studies with class III evidence and a limited number with class II evidence; currently, no class I evidence exists to guide recommendations for this subject. These data should be used when counseling patients regarding the probability of long-term maintenance of serviceable hearing following microsurgery for sporadic VSs. The greatest risk to hearing occurs upfront with surgery. If serviceable hearing is initially maintained, these results are generally durable. When including patients who lose useful hearing immediately following surgery, at 10 years, less than half of patients who begin with serviceable hearing will maintain useful hearing levels.
Observation
Question 7
What is the overall probability of maintaining serviceable hearing with conservative observation of vestibular schwannomas at 2 years, 5 years, and 10 years following diagnosis?
Target population
These recommendations apply to adults with small to medium-sized sporadic vestibular schwannomas who have documented serviceable hearing in the ipsilateral ear at time of diagnosis.
Recommendation
Level 3: Individuals who meet these criteria and are considering observation should be counseled that there is a high probability (>75% to 100%) of hearing preservation at 2 years, moderately high probability (>50% to 75%) of hearing preservation at 5 years, and moderately low probability (>25% to 50%) of hearing preservation at 10 years.
Question 8
Among patients with AAO-HNS class A or GR grade I hearing at baseline, what is the overall probability of maintaining serviceable hearing with conservative observation at 2 years and 5 years following diagnosis?
Target population
These recommendations apply to adults with small to medium-sized (<2 cm) sporadic vestibular schwannomas who have documented class A or GR grade I hearing in the ipsilateral ear at time of diagnosis.
Recommendation
Level 3: Individuals who meet these criteria and are considering stereotactic radiosurgery should be counseled that there is a high probability (>75% to 100%) of hearing preservation at 2 years, and moderately high probability (>50% to 75%) of hearing preservation at 5 years. Insufficient data were available to determine the probability of hearing preservation at 10 years for this population subset.
Question 9
What patient and tumor related factors influence progression to nonserviceable hearing during conservative observation?
Target population
These recommendations apply to adults with small to medium-sized (<2 cm) sporadic vestibular schwannomas who have documented serviceable hearing in the ipsilateral ear at time of diagnosis.
Recommendation
Level 3: Individuals who meet these criteria and are considering observation should be counseled regarding probability of successful hearing preservation based on the following prognostic data: the most consistent prognostic features associated with maintenance of serviceable hearing are good preoperative word recognition and/or pure tone thresholds with variable cut-points reported, as well as nongrowth of the tumor. Tumor size at the time of diagnosis, age, and sex do not predict future development of nonserviceable hearing during observation.
Study Selection
A total of 1307 studies were screened and assessed for eligibility, and 17 were included in the final review.2,19,62,69,92,114,139,142–151 Specific to this recommendation, only studies evaluating outcomes of hearing preservation following conservative observation with serial imaging, with a minimum of 20 patients, and with a median or mean of at least 2 years of follow-up are included.
Study Characteristics
Data extraction included study design, class of evidence, primary treatment modality, total number of patients, number of patients with serviceable hearing at time of observation commencement, study selection parameters, mean or median tumor size, mean or median follow-up, inclusion of NF2, inclusion of recurrent VSs, percentage of patients with serviceable hearing between 1 and 10 years, and prognostic features associated with development of nonserviceable hearing.
Risk of Bias and Study Limitations
Because all selected publications were either retrospective or nonrandomized prospective studies, there is a substantial risk of selection bias. Specific to conservative observation, this population is frequently older and includes smaller tumors at the time of diagnosis than patients selected for microsurgery or radiation.152 In addition, the definition of tumor growth or “failed” conservative management is extremely variable between studies. For example, some publications report progression of symptoms, including hearing, to denote failure; others specify an increase in tumor size or volume cutoff, most consistently ≥2 mm in greatest axial dimension compared to initial imaging.69,150
Results of Individual Studies
The key results of individual studies are outlined in Table 4 and are summarized within the guideline recommendations. In addition to the studies discussed earlier comparing conservative management to radiation therapy or microsurgery, several notable single-arm studies evaluating conservative management have been reported. The most robust data evaluating long-term hearing preservation with conservative observation comes from Copenhagen, Denmark, where a single centralized unit has evaluated virtually all newly diagnosed VSs in the country for more than 3 decades, and a substantial proportion of patients with tumors <2 cm are initially allocated to observation. In 2010, Stangerup et al19 evaluated the outcomes of 1144 patients who were initially managed with conservative observation. Within this group, 377 patients had a minimum of 5 years of follow-up, and 102 patients had at least 10 years. Overall, 249 of 455 (55%) patients who presented with AAO-HNS class A or B hearing maintained serviceable hearing at last follow-up, and when only evaluating those who presented with class A hearing, 81% (144/178 patients) maintained serviceable hearing at last follow-up. In 2008, Ferri et al148 reported the results of a prospective study where 123 patients with VSs were observed for a mean follow-up of 4.8 years. Of 56 patients who initially presented with serviceable hearing, 41 (73%) maintained useful hearing at last follow-up. The remaining single-arm studies evaluating conservative management had significantly fewer patients or shorter follow-up and will not be individually discussed beyond the evidence table summary.
Synthesis of Results
Class III evidence supports the conclusion that the risk of HL increases with time during conservative management. Similar to radiation therapy, the development of nonserviceable hearing is often protracted, continuing many years beyond diagnosis. When evaluating all patients with small to medium-sized sporadic VSs with serviceable hearing at the initiation of an observation period, 85% will maintain serviceable hearing at 2 years, 53% at 5 years, and 36% at 10 years. The 2 strongest prognostic factors for the development of nonserviceable hearing are tumor growth and poorer hearing at the beginning of observation.
Discussion
There were 2 literature reviews pertaining to VSs in the last 10 years that evaluated hearing preservation after conservative observation. In 2005, Smouha et al153 performed a meta-analysis literature review and evaluated a total of 21 studies comprising 1345 patients, with an average length of follow-up of 3.2 years (range 2.2–5 years). Of 1244 patients with adequate data, 43% demonstrated varying rates of growth.153 Data regarding audiologic outcome was available in 347 patients. Within this cohort, hearing was “preserved” in 49% and “lost” in 51%. In this study, rate of loss over time (ie, dB HL loss per year and SDS% loss per year) was not reported. In addition, data concerning hearing class were not described. In 2010, Sughrue et al154 analyzed the outcomes of 982 patients collected from 34 articles. Only publications that included patients with serviceable hearing at presentation were included, and “hearing preservation” was defined as having AAO-HNS class A-B or GR grade I-II at the end of follow-up. Over a range of follow-up between 26 and 52 months, the overall hearing preservation rate was 54%, which aligns with estimates derived from the current systematic review. The authors found that slower growth rate (≤2.5 mm/year) was associated with a greater probability of hearing preservation.
Summary
The evidence for this guideline was primarily drawn from studies with class III evidence and a limited number with class II evidence; currently no class I evidence exists to guide recommendations for this subject. These data should be used when counseling patients regarding the probability of long-term maintenance of serviceable hearing during conservative management of sporadic VSs. The risk of developing nonserviceable hearing is cumulative over time, and at 10 years, less than half of patients who begin with serviceable hearing will maintain useful hearing levels.
General Discussion
The current systematic review seeks to analyze the risk of developing nonserviceable hearing in patients who initially present with AAO-HNS class A or B or GR grade I or II hearing. The impetus for developing this guideline was to provide a frame of reference to assist clinicians in offering accurate and realistic counseling regarding the prospects of long-term serviceable hearing by modality. This guideline demonstrates that in the long run, the majority of patients develop nonuseful hearing in the ipsilateral ear either as a result of disease or as a consequence of treatment. The risk of HL with surgery is upfront; if useful hearing is initially preserved following surgery, the results appear to be durable in many cases, for at least 10 years. This is in contrast to radiation and conservative observation, where the initial risk to hearing is low; however, delayed loss is common and progressive over time. Therefore, in the short term, patients are most likely to maintain useful hearing following conservative management or contemporary low-dose radiation therapy. However, if progressive HL continues indefinitely in the latter 2 groups, which could be reasonably inferred from the current data, then the very long-term advantage may favor microsurgery, provided that hearing is initially preserved in a healthy proportion of patients undergoing surgery. Both the short- and long-term risks of HL should be considered, because most patients with VSs are diagnosed in their 40s to 60s and are expected to live several decades longer.
The remainder of the discussion primarily focuses on reviewing the only 2 studies offering class II evidence comparing radiosurgery and microsurgery,71,75 in addition to several recent literature reviews.155–157 In 2006, Pollock et al75 reported the first prospective, nonrandomized study comparing outcomes between 36 patients who received microsurgery and 46 patients who received radiosurgery. Preservation of serviceable hearing was greater for the radiosurgery arm than the microsurgical group at 3 months (77% vs 5%, P < .001), 1 year (63% vs 5%, P < .001), and last follow-up (63% vs 5%, P < 0001). A similar finding was reported when comparing the rate of AAO-HNS class A hearing between groups. Subsequently, in 2009, Myrseth et al71 reported the second prospective, nonrandomized study comparing outcomes of 63 patients who underwent Gamma Knife radiosurgery and 28 patients who underwent microsurgery. At both the 1- and 2-year time points, the Gamma Knife radiosurgery cohort had a statistically significantly greater proportion of patients with hearing preservation compared to the microsurgery group. In both studies, the Gamma Knife radiosurgery cohorts were older than the microsurgery groups; however, there was no difference in baseline tumor size. In the study by Pollock et al,75 the retrosigmoid approach was used in 69% of cases, while the retrosigmoid approach was used exclusively for patients who underwent microsurgery in the study by Myrseth et al71
In 2003, Yamakami et al157 published a large review comparing outcomes following radiation therapy (9 studies, 1475 patients), microsurgery (16 studies, 5005 patients), and conservative observation (13 studies, 903 patients). In total, 57% of 271 patients who received radiation treatment retained useful hearing following treatment, 36% of 1448 patients who underwent microsurgical resection with intent of hearing preservation, and 63% of 60 patients who were observed. Notably, a number of patients were treated with higher dose radiation parameters than what are commonly used today (average marginal dose of 14.5 Gy), and a proportion of patients underwent hearing preservation microsurgery despite having larger tumors.
In 2012, Maniakas and Saliba156 published a review comparing the outcomes of radiosurgery and conservative management in studies with a minimum of 5 years of follow-up. Reviewing 4 studies (147 patients) that met the inclusion criteria for conservative management, 58.5% of subjects had preservation of useful hearing at an average of 7.75 years. This was compared to a 73.3% rate of useful hearing preservation in a sample size of 382 patients from 7 studies, following stereotactic radiotherapy, after a mean follow-up of 6.4 years. Although this difference reached strong statistical significance, the authors concluded that the current literature does not provide enough evidence to make any definitive conclusions regarding differences in long-term hearing preservation with conservative management or radiation. They emphasized that more long-term studies, with homogenous data, are required. Notably, the results of this analysis differed quite substantially for radiation therapy compared to other reviews, and the number of analyzed patients was small. In 2003, Shin et al158 performed a literature review study evaluating neurotologic complications after radiosurgery compared to conservative management. The authors concluded that the probability of HL was much greater after radiosurgery (P < .05); however, detailed descriptions of study methodology pertaining to hearing classification and outcome were not presented.
This same year, Maniakas and Saliba155 published a second literature review comparing long-term hearing and tumor control outcomes between microsurgery and radiation therapy for small (<2 cm) VSs, requiring a minimum of 5 years of follow-up. Eight studies analyzing 410 cases were included in the stereotactic radiation population. The mean duration of follow-up was 6.9 years and 70.2% of patients had a useful hearing preservation outcome. This is compared to 7 studies with 77 patients who underwent microsurgery, including 38 who received retrosigmoid craniotomy and 39 who underwent middle fossa craniotomy. There was no statistical difference between surgical approaches, and the overall hearing preservation rate of 50.3% was seen at an average follow-up of 7.1 years. The authors concluded that stereotactic radiation therapy offered a greater probability of durable hearing preservation compared to microsurgery (P < .001). In 2000, Kaylie et al159 also performed a review comparing microsurgery and radiosurgery and found that the prevalence of hearing preservation was identical between modalities. Specifically, at a mean follow-up of 24 months, 44% of 599 patients who received microsurgery and 44% of 219 patients who received radiosurgery retained serviceable hearing following treatment.
In addition to the specific biases associated with individual treatment modalities, several general limitations of the VS hearing preservation literature warrant review. Many studies only provide the overall prevalence of hearing preservation at the median or mean study follow-up, and a significant number fail to present estimates at separate time points using time-to-event analysis (ie, Kaplan–Meier survival analysis), which is critical for interstudy comparison. Another important general limitation is the frequent lack of information regarding length of audiometric follow-up. In many studies, “follow-up” is marked by the most recent clinical evaluation or magnetic resonance imaging study and not always the most recent audiometric time point. Therefore, it is not always known whether a study reporting long-term outcomes is also including long-term audiometric data, unless this is specifically detailed. A third common limitation of the hearing preservation literature is the frequent lack of reported data concerning HL in the contralateral ear, which becomes an important consideration with longer follow-up. Age-related HL in the contralateral ear, particularly in the elderly, should be used to adjust rate estimates of disease-associated audiometric decline. For example, if a patient develops a 35-db HL loss in the tumor ear and a 15-dB HL loss in the nontumor ear over 15 years, only a loss of 20 dB in the tumor ear can be logically attributed to disease or treatment effects.
It is critical to realize that the current set of guidelines should not replace personal experience. In the words of Michael E. Glasscock, III, we should not simply quote the literature when counseling our patients regarding the rate of success or complication with surgery; but it is our responsibility to track and know our own outcomes. The rate of HL with conservative management is not dependent on the observer; however, the success of hearing preservation with surgery is at least partly driven by the technical skill and experience of the surgical team, and therefore may vary significantly between centers and surgeons. This point was highlighted by Mangham,160 who after reviewing hearing preservation results between 11 centers with a relatively high volume of VS microsurgery concluded that the surgical team accounted for more variability in hearing preservation outcome than the surgical approach. This also holds true to some extent with radiation therapy, where nuances of dose planning and cochlear shielding may influence long-term hearing preservation.48
Finally, we should not lose sight of the forest for the trees. Hearing preservation is only 1 of many factors that should be considered when counseling patients regarding potential treatment options. In addition, when considering the weighted impact of various disease- and treatment-related symptoms, other variables, including ongoing dizziness, headache, and facial paralysis, may be more burdensome to the patient, provided that the contralateral ear has good hearing.152,161,162 Ultimately, patient characteristics including age, health status, tumor size, hearing capacity (in both ears), occupational needs, and personal preference should all be considered. When analyzing all newly diagnosed VSs, less than half present with serviceable hearing, and a smaller percentage are eligible for hearing preservation treatment strategies.163 For example, tumors >2.5 cm in maximum posterior fossa dimension are most commonly allocated to surgery; however, in many centers, hearing preservation is not even attempted on a tumor this size even if useful hearing is present.10
Key Issues for Future Investigation
In addition to understanding the pattern of HL within individual treatment modalities, high-quality comparisons of hearing preservation between modalities is of paramount concern. The data acquired in the current systematic review demonstrates that many of the same features that predict a favorable outcome with one modality also confer a good outcome with another. For example, smaller tumor size, better hearing at baseline, and greater distance from the cochlea (which is related to cochlear dose with radiation therapy and fundal fluid cap with microsurgery) are associated with better outcomes whether managed by observation, surgery, or radiation. As a result of the great variability in outcomes reported by single-arm publications, and the significant selection biases present in nonrandomized multimodality studies, a well-designed prospective randomized study is required to answer this question. To date, there are only 4 nonrandomized prospective studies comparing treatment modalities; currently, no Class I evidence exists.69,71,75,92 Unfortunately, it is unlikely that a prospective randomized trial comparing outcomes between all 3 treatment modalities will ever materialize given a significant number of obstacles, including patient recruitment in a relatively rare condition, the enrollment numbers required to detect clinically meaningful differences, and significant practice disparities between many major centers making multicenter collaborations difficult. Such barriers were encountered by Myrseth et al,71 who had to abandon an initial plan to randomize enrollment as patients were unwilling to submit treatment allocation to chance. In addition, when examining long-term hearing preservation outcomes, clinicians are chasing a moving target. By the time long-term data have been acquired, the state of the field may have changed significantly from improvements in surgical technique, intraoperative eighth nerve neuromonitoring, or radiation dose planning paradigms.
Current mainstream strategies for treatment of single-sided deafness involve routing of sound to the contralateral good ear, either surgically through bone conduction (eg, BAHA) or via a hearing aid system (eg, CROS, BiCROS). While cochlear implants have been approved by the US Food and Drug Administration for use in the United States since 1985 for bilateral advanced sensorineural HL, it has been only recently that data have emerged regarding implantation in patients with VSs and other “retrocochlear pathology.” Several studies from within the last decade have demonstrated relatively promising outcomes for patients with NF2 or sporadic VSs.164–166 In this setting, the cochlear nerve must be anatomically intact, and ideally, patients should not have a prolonged duration of deafness. Compared to auditory brainstem implantation, cochlear implantation has a much greater probability of achieving open-set speech recognition.164 Currently, cochlear implantation is not approved by the US Food and Drug Administration for single-sided deafness; therefore, insurance companies do not routinely cover implantation for patients with VSs unless both ears have severe to profound sensorineural HL. As cochlear implantation for single-sided deafness becomes more mainstreamed, it is likely that a greater number of publications aimed at further defining the role of cochlear implantation in patients with sporadic VSs will be published.167
In recent years, there has been a trend toward maximizing functional outcomes, even at the expense of tumor control.17,168 Within the field of microsurgery, this has been clearly demonstrated through the use of subtotal resection with or without planned postoperative radiation therapy to reduce risk of facial neuropathy for medium and large VSs.169 While not common in the United States, some centers also consider using subtotal resection in an attempt to preserve functional hearing in patients with larger tumors and good preoperative hearing.170 Paralleling the microsurgical literature, radiosurgery dose de-escalation, using a marginal dose of ≤13 Gy, has now become standard at most centers in the United States.81,171 In addition, strategies aimed at minimizing radiation dose to the cochlea are now commonly used, which in some cases may result in undertreatment of the lateral tumor margin in the fundus of the internal auditory canal.48 The preliminary results of these strategies appear promising; however, long-term follow-up is required to determine durability of tumor control and long-term risk of HL as a result of treatment or tumor recurrence.
A final key area of ongoing and future study is the use of medical therapy for prevention or salvage of disease- or treatment-related hearing deterioration. Therapies including topical and systemic calcium channel blockers (eg, nimodipine) and vasodilators (eg, Papaverine) might demonstrate some utility as an adjunct for hearing preservation microsurgery, where vasospasm of labyrinthine vasculature has been proposed as a mechanism of HL.172–174 Glucocorticoid therapy is frequently used perioperatively, but has also been applied to cases of sudden sensorineural HL with observed VSs and as an adjunct to radiation treatment.65,175 Recent studies have demonstrated that aspirin use may have a protective effect against tumor growth in patients with observed, sporadic VSs. Additional research will be needed to validate these findings and to ascertain any benefit with regard to hearing preservation.176 Finally, anti–vascular endothelial growth factor therapy for patients with NF2 has demonstrated dramatic results in select individuals.177,178 Future studies will be required to define the role of anti–vascular endothelial growth factor therapy in mitigating HL with treatment or from natural tumor progression.
Conclusions
A systematic review of the existing evidence was performed to formulate a series of clinical guidelines clarifying the probability of hearing preservation at different time points following treatment and to elucidate the key prognostic features that predict hearing deterioration. These data demonstrate that consistent and durable hearing preservation in sporadic VSs remains an elusive goal. Most patients eventually develop nonserviceable hearing as a result of disease or treatment. Class III and limited Class II evidence suggests that there is not one clear advantage of one modality over another with regard to long-term hearing preservation. At 10 years following treatment, more than half of patients with baseline serviceable hearing will progress to nonuseful hearing levels regardless of treatment modality.
Conflict of Interest (COI)
The Vestibular Schwannoma Guidelines Task Force members were required to report all possible COIs prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of Task Force members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methods manuscript (here).
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient’s physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons and the Tumor Section of the Congress of Neurological Surgeons and the American Association of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document.
Acknowledgments
The authors acknowledge the Congress of Neurological Surgeons Guidelines Committee for its contributions throughout the development of the guideline and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Guidelines Committee for its review, comments, and suggestions throughout peer review, as well as Trish Rehring, MPH, CHES, and Mary Bodach, MLIS, for their assistance. Throughout the review process, the reviewers and authors were blinded from one another. At this time, the guidelines task force would like to acknowledges the following individual peer reviewers for their contributions: Sepideh Amin-Hanjani, MD, D. Ryan Ormond, MD, Andrew P. Carlson, MD, Kimon Bekelis, MD, Stacey Quintero Wolfe, MD, Chad W. Washington, MD, Cheerag Dipakkumar Upadhyaya, MD, and Mateo Ziu, MD.
Figures
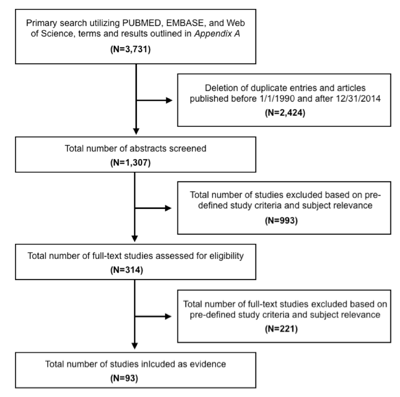
Figure 1. Article flow chart.
Table 1. Primary search strategy, results, and initial pruning
|
ENDNOTE PUBMED (NLM), searched on May 9th 2015:
|
|
Search 1: All Fields, Contains “acoustic neuroma” AND All Fields, Contains “hearing preservation”
Results: 788
|
|
Search 2: All Fields, Contains “vestibular schwannoma” AND All Fields, Contains “hearing preservation”
Results: 434
|
|
Search 3: All Fields, Contains “acoustic neuroma” AND All Fields, Contains “audiometric”
Results: 164
|
|
Search 4: All Fields, Contains “vestibular schwannoma” AND All Fields, Contains “audiometric”
Results: 94
|
|
Search 5: All Fields, Contains “acoustic neuroma” AND All Fields, Contains “hearing” AND “predictors”
Results: 24
|
|
Search 6: All Fields, Contains “vestibular schwannoma” AND All Fields, Contains “hearing” AND “predictors”
Results: 21
|
|
Total: 1525
|
|
ENDNOTE EMBASE, searched on May 9th, 2015:
|
|
Search 1: Abstract, Contains “acoustic neuroma” AND Abstract, Contains “hearing preservation”
Results: 170
|
|
Search 2: Abstract, Contains “vestibular schwannoma” AND Abstract, Contains “hearing preservation”
Results: 221
|
|
Search 3: Abstract, Contains “acoustic neuroma” AND Abstract, Contains “audiometric”
Results: 59
|
|
Search 4: Abstract, Contains “vestibular schwannoma” AND Abstract, Contains “audiometric”
Results: 55
|
|
Search 5: Abstract, Contains “acoustic neuroma” AND Abstract, Contains “hearing” AND Abstract, Contains “predictors”
Results: 3
|
|
Search 6: Abstract, Contains “vestibular schwannoma” AND Abstract, Contains “hearing” AND Abstract, Contains “predictors”
Results: 16
|
|
Total: 524
|
|
ENDNOTE Web of Science, searched on May 9th, 2015:
|
|
Search 1: Title/Keywords/Abstract, Contains “acoustic neuroma” AND Title/Keywords/Abstract, Contains “hearing preservation”
Results: 785
|
|
Search 2: Title/Keywords/Abstract, Contains “vestibular schwannoma” AND Title/Keywords/Abstract, Contains “hearing preservation”
Results: 676
|
|
Search 3: Title/Keywords/Abstract, Contains “acoustic neuroma” AND Title/Keywords/Abstract, Contains “audiometric”
Results: 94
|
|
Search 4: Title/Keywords/Abstract, Contains, Contains “vestibular schwannoma” AND Title/Keywords/Abstract, Contains, Contains “audiometric”
Results: 68
|
|
Search 5: Title/Keywords/Abstract, Contains, Contains “acoustic neuroma” AND Title/Keywords/Abstract, Contains, Contains “hearing” AND Title/Keywords/Abstract, Contains, Contains “predictors”
Results: 27
|
|
Search 6: Title/Keywords/Abstract, Contains, Contains “vestibular schwannoma” AND Abstract, Contains “hearing” AND Title/Keywords/Abstract, Contains, Contains “predictors”
Results: 32
|
|
Total: 1682
|
|
Summary of Primary Search
Combined from 3 database searches, total of 3731 candidate articles
Deleted articles published before 1/1/1990 and after 12/31/2014
Deleted all duplicate articles
Total number of candidate articles after primary search = 1307
|
Table 2.Radiation therapy
|
Author/Year
|
Study Description
|
Data Class
|
Results and Conclusion
|
|
Puataweepong et al, 2014
|
Objective: To analyze VS treatment outcomes after SRS, HSRT, and CSRT using a LINAC-based system. Hearing preservation, complications, and tumor control were compared.
Design: Retrospective case series extracted from a prospectively maintained database, single institutional experience
Number of patients: 139 patients in total, 39 treated with SRS, 79 with HSRT, and 28 with CSRT. At baseline 49 had serviceable hearing overall; 4 (10%) SRS patients had serviceable hearing at baseline, 33 (42%) treated with HSRT and 12 (43%) CSRT. Dose strategies variable within subgroups.
Follow-up: Median 61 months.
|
III
|
Results: Overall, 76% (10/13) of patients with pretreatment GR grade I hearing, and 83% (30/36) with GR grade II hearing maintained serviceable hearing. Overall hearing preservation rates at 1, 2, and 5 years were 90%, 84%, and 80% respectively. The 5-year hearing preservation rates after SRS, HSRT and CSRT were 75%, 87% and 63% respectively (P = .35).
Conclusion: There is no statistically significant difference in tumor control, hearing preservation, and complications following SRS, HSRT, and CSRT for VSs. The authors conclude that HSRT may be better than CSRT for patients with pretreatment serviceable hearing given the shorter treatment times.
|
|
Kranzinger et al, 2014
|
Objective: To evaluate HSRT for treatment of vestibular schwannomas with a focus on tumor control and hearing preservation
Design: Prospective cohort using 7 × 4 Gy ICRU dose protocol, single institution experience
Number of patients: 29 patients total, 23 with pretreatment serviceable hearing, 21 of the latter with serial posttreatment audiologic follow-up
Follow-up: Median 71.3 months (audiometric follow-up).
|
II
|
Results: The 5-year actuarial rate of hearing preservation was 50%. Patients with pretreatment speech discrimination score of 90–100% were much more likely to maintain serviceable hearing than those with lower scores (P = .002)
Conclusion: Posttreatment tumor swelling is common. The rate of hearing decline following HSRT is only minimally greater than the natural history of VS-related hearing loss.
|
|
Jacob et al, 2014
|
Objective: To evaluate association between volumetric cochlear dose and preservation of useable hearing after Gamma Knife radiosurgery. To assess intra- and interobserver reliability in determining modiolar point dose and to review clinical significance of cochlear dose with regard to SRS planning.
Design: Retrospective case series, single institution experience
Number of patients: 59 patients with pretreatment serviceable hearing
Follow-up: Mean 25.2 months.
|
III
|
Results: 21 (36%) developed nonserviceable hearing at a mean of 2.2 years following radiosurgery. Univariate predictors of nonserviceable hearing included pretreatment pure tone thresholds, speech discrimination scores, AAO-HNS hearing class, marginal dose, and mean dose to the cochlear volume. Multivariate analysis revealed that only pure tone thresholds were predictive after accounting for baseline differences.
Conclusion: Cochlear dose is one of many variables associated with loss of serviceable hearing.
|
|
Tsai et al, 2013
|
Objective: To evaluate tumor control and hearing preservation following LINAC-based CyberKnife radiation therapy (marginal dose 18 Gy over 3 sessions, with a 72–90% isodose line) for VSs. To evaluate prognostic factors of hearing loss.
Design: Retrospective case series, 2 separate medical centers.
Number of patients: 117 total, 65 with pretreatment serviceable hearing
Follow-up: Mean 64.5 months audiometric follow-up.
|
III
|
Results: 81.5% (53/65) maintained serviceable hearing at a mean follow-up of 64.5 months. Larger tumor volume and smaller cochlear volumes were associated with hearing loss after radiation therapy.
Conclusion: LINAC-based CyberKnife treatment of vestibular schwannomas provides excellent tumor control and hearing preservation. Larger tumor size, poorer pretreatment hearing levels, and smaller cochlear volume are associated with poorer hearing preservation following radiation treatment.
|
|
Vivas et al, 2013
|
Objective: To evaluate hearing, tinnitus, balance, and tumor control outcomes after LINAC-based CyberKnife radiosurgery for VSs. Treatment plan included 18 Gy administered over 3 equal fractions to the 80% isodose line separated by at least 48 hours.
Design: Retrospective case series, single institution
Number of patients: 73 patients total, 28 with serviceable hearing prior to treatment
Follow-up: Mean 40 months.
|
III
|
Results: Of patients with serviceable hearing before CyberKnife, 53.5% (15/28) maintained serviceable hearing at 3 years of follow-up. Of patients with pretreatment AAO-HNS Class A hearing, 77% (10/13) maintained serviceable hearing.
Conclusion: LINAC-based CyberKnife provides similar rates of tumor control and hearing preservation compared to other forms of radiosurgery.
|
|
Breivik et al, 2013
|
Objective: To evaluate the effect of Gamma Knife radiosurgery on growth and hearing compared to conservatively managed vestibular schwannomas with extracanalicular extension
Design: Prospective cohort study, single institution experience.
Number of patients: 237 total, 113 receiving radiosurgery, 124 conservatively managed. 114 patients had serviceable hearing prior to radiosurgery.
Follow-up: Mean 55 months.
|
II
|
Results: Serviceable hearing was lost in 76% (54/71) of observed tumors and 64% (34/53) of tumors that received radiosurgery (not a statistically significant difference).
Conclusion: Gamma Knife radiosurgery reduces the tumor growth rate compared to conservatively managed tumors. Hearing is lost at a similar rate between groups. Symptoms and quality of life are not different between groups.
|
|
Massager et al, 2013
|
Objective: To evaluate dose of radiation to the cochlea during Gamma Knife radiosurgery (marginal dose 12 Gy) for VSs and to determine associations between treatment variables and hearing preservation
Design: Retrospective case series, single institution experience.
Number of patients: 82 total, 60 with pretreatment serviceable hearing
Follow-up: Median 2 years.
|
III
|
Results: 65% (39/60) of patients with serviceable hearing maintained serviceable hearing at last follow-up. Cochlear dose was strongly associated with hearing deterioration.
Conclusion: Cochlear dose is strongly associated with hearing deterioration following Gamma Knife radiosurgery for VSs. At a median of 2 years following radiosurgery, 65% of patients will maintain serviceable hearing.
|
|
Lunsford et al, 2013
|
Objective: To evaluate tumor control, hearing preservation, and cranial nerve outcomes following Gamma Knife radiosurgery (median marginal dose 13 Gy) for treatment of VSs.
Design: Retrospective case series, single institution experience.
Number of patients: 829 total, number with pretreatment serviceable hearing not specified.
Follow-up: Not specified.
|
III
|
Results: The 5-year actuarial rates of hearing level preservation and speech discrimination preservation were 69% and 86%, respectively, for tumors that were treated with ≤13 Gy at the tumor margin.
Conclusion: Gamma Knife radiosurgery provides low risk, effective treatment for VSs. Hearing preservation is possible in a large percentage of patients using modern dose planning.
|
|
Litre et al, 2013
|
Objective: To evaluate long-term outcomes of LINAC-based FRST (50.4 Gy) for VSs
Design: Prospective cohort, single institution experience.
Number of patients: 155 total, 61 with serviceable pretreatment hearing
Follow-up: Median 60 months.
|
II
|
Results: 54% (33/61) of patients with pretreatment serviceable hearing maintained serviceable hearing at last follow-up. Among patients with pretreatment GR grade I hearing, 63% maintained serviceable hearing at last follow-up.
Conclusion: LINAC-based FSRT is safe and effective for treatment of vestibular schwannomas. Compared to radiosurgery, there are no contraindications to use, including patients with larger tumor size.
|
|
Kim et al, 2013
|
Objective: To evaluate prognostic factors for hearing preservation for sporadic intracanalicular VSs following Gamma Knife radiosurgery (mean marginal dose 12.2 Gy)
Design: Not specified, but appears to be retrospective, single institution experience
Number of patients: 60, all with serviceable hearing prior to radiosurgery
Follow-up: Median 62 months.
|
III
|
Results: Actuarial hearing preservation rates at 1, 2, and 5 years following radiosurgery were 70%, 63%, and 55%, respectively. Transient posttreatment tumor expansion was the strongest predictor of hearing deterioration.
Conclusion: Among patients with intracanalicular VSs, transient tumor expansion following radiosurgery is associated with an increased risk of hearing deterioration. At 5 years, 55% of patients with serviceable hearing before radiosurgery will maintain serviceable hearing.
|
|
Hayden Gephart et al, 2013
|
Objective: To analyze hearing preservation following LINAC-based CyberKnife radiosurgery (18 Gy, 3 equal fractions, median marginal dose to the 75–85% isodose line) for treatment of VSs. To analyze associations between cochlear radiation dose and hearing preservation.
Design: Retrospective case series, single institution experience
Number of patients: 94 patients with GR grade I or II before treatment
Follow-up: Mean 2.4 years audiometric follow-up.
|
III
|
Results: Overall 74% (70/94) of patients with GR grade I or II maintain serviceable hearing at a mean of 2.4 years following CyberKnife radiosurgery. Higher radiation dose and larger irradiated cochlear volume were associated with hearing deterioration.
Conclusion: Following CyberKnife radiosurgery, 74% of patients maintained serviceable hearing. Higher radiation dose and larger irradiated cochlear volume were significantly associated with risk of hearing loss.
|
|
Combs et al., 2013
|
Objective: To assess long-term tumor control, treatment toxicity, and hearing preservation following fractionated radiotherapy (median dose of 57.6 Gy, 1.8 Gy per fraction, 5 fractions per week, 90% isodose line) and radiosurgery (median marginal dose of 13 Gy to the 80% isodose) for treatment of VSs
Design: Retrospective case series with cross-sectional survey assessing symptom control and quality of life, single institution experience
Number of patients: 248 total; fractionated radiotherapy in 216 and radiosurgery in 32. 116 patients had pretreatment GR grade I or II
Follow-up: Median 92 months.
|
III
|
Results: Among patients presenting with serviceable hearing prior to radiation treatment, the 1-, 3-, 5-, and 10-year rates of hearing preservation were 89.7%, 84.7%, 76.5%, and 68.6%, respectively. After 10 years of follow-up, hearing deterioration continued in both groups.
Conclusion: Fractionated and single-fraction radiotherapy for VSs provide high rates of long-term tumor control with favorable rates of hearing preservation. The risk of hearing deterioration was not different between fractionated and single-fraction delivery when examining the SRS group that received ≤13 Gy to the tumor margin.
|
|
Champ et al, 2013
|
Objective: To report tumor control, functional outcome, and hearing preservation with reduced dose LINAC-based FSRT (total 46.8 Gy in 1.8-Gy fractions)
Design: Retrospective case series, single institution experience.
Number of patients: 154 total, 87 serviceable hearing prior to treatment
Follow-up: Median 35 months.
|
III
|
Results: Cumulative rate of hearing preservation was 67% (58/87). When specifically analyzing the group with pretreatment GR grade I, the overall rate of hearing preservation was 82%. Univariate and multivariate analysis revealed that pretreatment hearing class and cochlear dose where strong predictors of hearing preservation following radiation treatment.
Conclusion: Reduced dose FSRT provides excellent hearing preservation, tumor control, and limited toxicity.
|
|
Carlson et al, 2013
|
Objective: To describe the pattern and timing of hearing loss following Gamma Knife radiosurgery (12–13 Gy marginal dose) for VSs
Design: Retrospective case series, single institution experience.
Number of patients: 44 total, all with serviceable hearing prior to treatment and at least 5 years of audiometric follow-up
Follow-up: Median 9.3 years.
|
III
|
Results: 18% (36/44) of patients maintained serviceable hearing at last follow-up. Kaplan–Meier estimates of nonserviceable hearing at 1, 3, 5, and 10 years following radiation were 80%, 55%, 48%, and 23%. Pretreatment tumor size and pretreatment pure tone average were strong predictors of hearing deterioration following radiosurgery on multivariable analysis.
Conclusion: Durable hearing preservation a decade following stereotactic radiosurgery occurs in less than a quarter of patients. Pretreatment hearing capacity and tumor size predict development of nonserviceable hearing.
|
|
Baschnagel et al, 2013
|
Objective: To determine the rate of hearing preservation after Gamma Knife radiosurgery (median marginal dose of 12.5 Gy) for treatment of VSs. To determine the association between cochlear dose and development of nonserviceable treatment following radiation.
Design: Retrospective case series, single institution experience.
Number of patients: 40 patients, all with serviceable hearing prior to radiation therapy
Follow-up: Median 35 months.
|
III
|
Results: The 1-, 3- and 5-year rates of hearing preservation following radiosurgery were 93%, 77%, and 74%, respectively. Cochlear dose and volume of cochlea irradiated were associated with development of nonserviceable hearing.
Conclusion: A cochlear dose <3 Gy is associated with higher hearing preservation rates following Gamma Knife radiosurgery.
|
|
Yomo et al, 2012
|
Objective: To compare the rate of hearing loss during initial conservative observation to results after Gamma Knife radiosurgery (marginal dose 12.1 Gy) among patients with VSs.
Design: Retrospective case series using within-subject control, single institution experience.
Number of patients: 154 total, 105 with serviceable hearing prior to radiosurgery
Follow-up: Mean 52 months following treatment.
|
III
|
Results: 58% of the patients with serviceable hearing prior to Gamma Knife radiosurgery retained serviceable hearing at the time of last follow-up
Conclusion: The AHDR was less severe following radiosurgery than following the initial period of observation. Cochlear dose is a prognostic factor for development of nonserviceable hearing.
|
|
Sun et al, 2012
|
Objective: To assess long-term clinical outcomes following Gamma Knife radiosurgery (14 Gy or less to the margin) for treatment of sporadic VSs.
Design: Retrospective case series, single institution experience.
Number of patients: 190 total, but only 22 with serviceable hearing before treatment
Follow-up: Median 109 months.
|
III
|
Results: 86% (19/22) of patients with pretreatment serviceable hearing maintained serviceable hearing following radiation.
Conclusion: Using low-dose (≤14 Gy to the margin) Gamma Knife radiosurgery provides tumor control and minimal cranial nerve injury in sporadic VSs. Long-term follow-up is required because of the risk of delayed tumor recurrence.
|
|
Rasmussen et al, 2012
|
Objective: To evaluate long-term tumor control and hearing preservation using FRST (54 Gy in 27–30 fractions during 5.5–6.0 weeks) for VSs and to compare to an untreated control group. To assess the relationship between dose to the cochlea and rate of hearing preservation.
Design: Retrospective case-control study, single institution experience.
Number of patients: 42 total, 21 with pretreatment serviceable hearing; 409 historical controls
Follow-up: Median 5 years.
|
II
|
Results: 38% (8/21) of patients maintained serviceable hearing at 2 years following FRST, and none maintained serviceable hearing at 10 years. The hearing preservation rates in the control group were 1.8 times better than the treatment group at 2 years. Cochlear dose predicts deterioration of speech reception threshold.
Conclusion: Fractionated stereotactic radiotherapy accelerates hearing loss over the natural history. Radiation dose to the cochlea predicts loss of hearing thresholds.
|
|
Han et al, 2012
|
Objective: To identify prognostic factors for hearing preservation among patients who undergo Gamma Knife radiosurgery (median marginal dose 12 Gy) for sporadic VSs.
Design: Does not specify but appears to be a retrospective case series, single institution experience.
Number of patients: 119, all with pretreatment serviceable hearing.
Follow-up: Mean 55.2 months.
|
III
|
Results: In multivariate analysis, pretreatment pure tone average and ABR interlatency waves I–V were significant independent prognostic factors for hearing preservation. At last follow-up, 43% of patients lost serviceable hearing. The actuarial rates of hearing preservation at 12, 24, 36, and 60 months were 68.5%, 62.5%, 59.9%, and 56.2%, respectively, after radiosurgery.
Conclusion: Pretreatment pure tone average score and ABR interlatency waves I–V were useful to predict hearing preservation with Gamma Knife radiosurgery.
|
|
Kopp et al, 2011
|
Objective: To assess tumor control- and treatment-related side effects of LINAC-based stereotactic radiosurgery (12 Gy to 100% isodose line) and fractionated radiotherapy (54 Gy at 1.8 Gy per fraction) for treatment of VSs.
Design: Prospective nonrandomized cohort study, single institution experience.
Number of patients: 115 total including 47 received stereotactic fractionated radiation and 68 received radiosurgery. 39 patients had serviceable hearing prior to radiosurgery and 33 had serviceable hearing prior to fractionated radiotherapy.
Follow-up: Mean 32.1 months for fractionated cohort and 30.1 months for single-fraction cohort.
|
II
|
Results: At a mean of approximately 30 months following treatment, 85% of patients maintained serviceable hearing following radiosurgery, and 79% after stereotactic fractionated radiotherapy.
Conclusion: High tumor control and a low rate of side effects occurred following fractionated and single-fraction stereotactic radiation for treatment of VSs.
|
|
Kim et al, 2011
|
Objective: To evaluate efficacy of corticosteroids on acute hearing loss following Gamma Knife radiosurgery for VSs. To evaluate for prognostic factors for hearing preservation.
Design: Prospective cohort with comparison to historical controls, single institution experience.
Number of patients: 41, all with serviceable pretreatment hearing.
Follow-up: Median 25 months.
|
III
|
Results: 61% (25/41) of patients maintained serviceable hearing following radiation therapy. The actuarial hearing preservation
was 75.2% at 1 year, 60.2% at 2 years, and 54.7% at 3 years.
Conclusion: Steroid therapy may improve acute hearing loss following stereotactic radiosurgery for VSs.
|
|
Hasegawa et al, 2011
|
Objective: To evaluate hearing preservation rates and factors associated with hearing preservation following Gamma Knife radiosurgery (12 Gy median dose to the margin) for treatment of VSs
Design: Retrospective case series, single institution experience.
Number of patients: 117, all with pretreatment serviceable hearing.
Follow-up: Median 38 months of audiometric follow-up.
|
III
|
Results: Actuarial 3-, 5-, and 8-year hearing preservation rates were 55%, 43%, and 34%, respectively. Actuarial 3- and 5-year hearing preservation rates were 71% and 64% in patients with pretreatment GR grade I hearing.
Conclusion: Gamma Knife radiosurgery is an effective alternative to surgery for treatment of small to medium-sized VSs. Pretreatment hearing class and cochlear radiation dose are associated with hearing deterioration following radiation.
|
|
Hansasuta et al, 2011
|
Objective: To evaluate outcomes following fractionated stereotactic radiosurgery (CyberKnife, 18 Gy divided into 3 sessions) for treatment of VSs.
Design: Retrospective case series, single institution experience.
Number of patients: 383 total, 200 with pretreatment serviceable hearing.
Follow-up: Median 3.0 years audiometric follow-up.
|
III
|
Results: Overall, 76% (151/200) of patients maintained serviceable hearing following treatment. Smaller tumor volume was associated with higher hearing preservation rates.
Conclusion: CyberKnife radiosurgery (18 Gy; 3 sessions) provides excellent tumor control and promising hearing preservation rates, with minimal risk of facial and trigeminal nerve injury.
|
|
Collen et al, 2011
|
Objective: To evaluate and compare outcomes after LINAC-based stereotactic radiosurgery (median marginal dose of 12.5 Gy to the 80% isodose line) and fractionated radiotherapy (10 fractions of 3 to 4 Gy or 25 fractions of 2 Gy) for VSs
Design: Retrospective case series, single institution experience.
Number of patients: 119 total, including 78 with single fraction and 41 with fractionated radiation. 35 with single-fraction and 19 with fractionated radiation treatment had serviceable hearing prior to radiation.
Follow-up: Median 62 months.
|
III
|
Results: Overall 4-year rate of preservation of serviceable hearing was 68%, 59% after single fraction, and 82% after fractionated treatment (P = .089). Overall, the 1-, 2- and 4-year hearing preservation rates were 87%, 81%, and 68%, respectively.
Conclusion: LINAC-based radiotherapy provides good tumor control and clinical outcomes in small to medium-sized VSs. Treatment of larger tumors with radiation remains challenging.
|
|
Regis et al, 2010
|
Objective: To compare tumor control and hearing outcomes between patients receiving conservative management and upfront radiosurgery (marginal dose 12 Gy) for treatment of VSs.
Design: Prospective cohort study, single institution experience.
Number of patients: 47 receiving observation (31 with serviceable hearing) and 34 receiving radiosurgery, all had functional hearing.
Follow-up: Median 34.7 months.
|
II
|
Results: Serviceable hearing preservation in the observation group at 3, 4, and 5 years was 75%, 52%, and 41%, respectively. Serviceable hearing preservation in the radiosurgery group at 3, 4, and 5 years was 77%, 70%, and 64%, respectively
Conclusion: Conservative treatment is associated with an increased risk of tumor growth and loss of serviceable hearing compared to upfront radiosurgery.
|
|
Tamura et al, 2009
|
Objective: To evaluate long-term hearing preservation after radiosurgery (median marginal dose 12 Gy) for patients with VSs and GR grade I hearing prior to treatment.
Design: Not specified, but appears to be a retrospective, single institution experience.
Number of patients: 74, all with GR grade I hearing before treatment.
Follow-up: Median 48 months.
|
III
|
Results: Serviceable hearing was maintained in 70% of patients at 8 years and beyond. Factors associated with hearing preservation included initial symptoms, tumor size, dose to cochlea, age (cut point 50 years), and IAC depth of penetration
Conclusion: Probability of serviceable hearing preservation following radiosurgery for VSs in patients with GR grade I hearing is high. Factors including age, initial symptoms, and dose to the cochlea predict risk of hearing loss.
|
|
Myrseth et al, 2009
|
Objective: To compare treatment-associated morbidity of radiosurgery (12 Gy to margin) and microsurgery for patients with VSs.
Design: Prospective nonrandomized cohorts, single institution experience.
Number of patients: 63 radiosurgery (25 with serviceable hearing), 28 microsurgery (13 with serviceable hearing)
Follow-up: Mean 2 years.
|
II
|
Results: No patients maintained serviceable hearing at 1 and 2 years after microsurgery, while 76% and 68% of patients maintained serviceable hearing at 1 and 2 years following radiosurgery.
Conclusion: Better facial nerve outcomes and hearing outcomes are achieved with radiosurgery compared to microsurgery for VSs.
|
|
Lasak et al, 2008
|
Objective: To evaluate hearing outcomes for patients with unilateral VSs who received Gamma Knife radiosurgery (12-13 Gy to margin). To determine if cochlear dose affects hearing outcomes.
Design: Retrospective case series, single institution experience.
Number of patients: 33 total, 10 with pretreatment AAO-HNS class A or B hearing.
Follow-up: Median audiometric follow-up of 24 months.
|
III
|
Results: At last follow-up, 9 of 10 patients maintained serviceable hearing. Six of 10 with AAO-HNS class A or B retained their original hearing classification. Cochlear dose was associated with hearing loss.
Conclusion: Pure tone average was significantly worse at 2 years following radiosurgery. Dose to the cochlea significantly affects hearing preservation outcomes.
|
|
Thomas et al, 2007
|
Objective: To determine hearing preservation rates and hearing preservation prognostic factors following FSRT (45 Gy in 25 fractions to the 90% isodose line) for VSs.
Design: Prospective cohort study, single institution experience.
Number of patients: 34 total, 33 with GR grade I or II hearing before treatment.
Follow-up: 36.5 months.
|
III
|
Results: The 2- and 3-year actuarial rates of serviceable hearing preservation were both 63%. Radiation dose to the cochlea was the only significant predictor of hearing deterioration.
Conclusion: Radiation dose to the cochlea is strongly predictive of hearing loss following FSRT for VSs.
|
|
Chopra et al, 2007
|
Objective: To evaluate long-term clinical outcomes of Gamma Knife radiosurgery (12-13 Gy marginal dose) for treatment of unilateral VSs.
Design: Retrospective case series, single institution experience.
Number of patients: 216 total, 106 with serviceable hearing prior to treatment.
Follow-up: Median 68 months.
|
III
|
Results: 56.6% (60/106) of patients with serviceable hearing maintained serviceable hearing at last follow-up. The 10-year actuarial preservation rate was 44.5%. Treatment volume was the only variable associated with preservation of hearing class.
Conclusion: Gamma Knife radiosurgery with 12–13 Gy to the tumor margin provides high rates of long-term tumor control and cranial nerve preservation.
|
|
Pollock et al, 2006
|
Objective: Comparison of tumor control and functional outcomes between patients receiving microsurgery and radiosurgery (mean dose to margin 12.2 Gy) for VSs.
Design: Prospective nonrandomized cohort study, single institution experience.
Number of patients: 82 total, 36 receiving microsurgery (22 with serviceable hearing before treatment), 46 receiving radiosurgery (30 with serviceable hearing before treatment)
Follow-up: Mean 42 months.
|
II
|
Results: Preservation of serviceable hearing at 1 year and last follow-up for the microsurgery cohort was 5% for both time points. Preservation of serviceable hearing at 1 year and last follow-up for radiosurgery was 63% for both time points (P < .01)
Conclusion: Early outcomes are better for radiosurgery compared to microsurgery for <3 cm unilateral VSs.
|
|
Massager et al, 2006
|
Objective: To evaluate association between hearing preservation and volumetric and dosimetric parameters of radiosurgery (marginal dose 12 Gy) for treatment of VSs.
Design: Retrospective case series, single institution experience.
Number of patients: 82 total, 62 with serviceable hearing before treatment.
Follow-up: Median 2 years.
|
III
|
Results: 65% (39/60) of patients with serviceable hearing before radiosurgery maintained serviceable hearing at last follow-up. Radiation dose to the cochlea and intracanalicular tumor volume are associated with hearing preservation following treatment.
Conclusion: Advise direct treatment for patients with serviceable hearing and <100 mm3 intracanalicular volume. For patients with larger intracanalicular volumes, dose reduction to the meatal tumor should be considered with movement of the maximum dose toward the extracanalicular portion of tumor.
|
|
Paek et al, 2005
|
Objective: To evaluate rate of hearing preservation and to determine prognostic factors following Gamma Knife radiosurgery (12 Gy to margin) for VSs.
Design: Prospective cohort study, single institution experience.
Number of patients: 25, all with serviceable hearing.
Follow-up: Median 49 months.
|
III
|
Results: 52% (13/25) of patients maintained serviceable hearing following radiosurgery. 35% (9/25) retained their pretreatment GR hearing class. Maximum dose to the cochlear nucleus was the only factor associated with hearing deterioration.
Conclusion: Improvements in radiation delivery are needed to prevent hearing deterioration in the first 6 months following radiation.
|
|
Hasegawa et al, 2005
|
Objective: To evaluate long-term outcomes using Gamma Knife radiosurgery for treatment of VSs (mean 13.2 Gy to the tumor margin).
Design: Retrospective case series, single institution experience.
Number of patients: 317, 90 with serviceable hearing before treatment and posttreatment audiometric follow-up.
Follow-up: Median 7.8 years.
|
III
|
Results: The hearing preservation rate was 68% (50/74) in patients that received a marginal dose of ≤13 Gy. The rate of hearing preservation was significantly poorer in patients treated with higher dose plans.
Conclusion: Radiosurgery provides safe and effective treatment and good functional outcomes for selected patients beyond 5 years of follow-up.
|
|
Combs et al, 2006
|
Objective: To evaluate the effectiveness and long-term outcome of stereotactic radiosurgery for VSs (median single marginal dose of 13 Gy, 80% isodose line).
Design: Prospective cohort, single institution experience.
Number of patients: 26 with serviceable hearing prior to treatment.
Follow-up: Not specified.
|
III
|
Results: Hearing preservation rate for patients with useful hearing before radiation therapy was 55% at 9 years.
Conclusion: Stereotactic radiosurgery results in good tumor control and low cranial nerve toxicities. Radiosurgery should be used with smaller lesions.
|
|
Combs et al, 2005
|
Objective: To evaluate long-term outcome and toxicity of FRST (median dose 57.6, median single fractions of 1.8 Gy, 5 per week) for treatment of VSs.
Design: Prospective cohort, single institution experience.
Number of patients: 106 total, 55 with serviceable hearing prior to treatment.
Follow-up: Median 48.5 months.
|
III
|
Results: Actuarial hearing preservation in patients who presented with serviceable hearing was 98% at 2 and 5 years.
Conclusion: Fractionated stereotactic radiotherapy is safe and efficacious for treatment of VSs, with mild toxicity with regard to hearing loss and cranial nerve function.
|
|
Flickinger et al, 2004
|
Objective: To define tumor control and clinical outcomes following Gamma Knife radiosurgery (12–13 Gy marginal dose) for VSs.
Design: Retrospective review, single institution experience.
Number of patients: 313 total, 246 had serviceable hearing prior to treatment.
Follow-up: Median 24 months.
|
III
|
Results: Serviceable hearing was preserved in 79% (218/246) of patients. None of the variables tested correlated with decline in hearing level.
Conclusion: Radiosurgery using 12–13 Gy to the tumor margin for treatment of VSs provides high rates of tumor control and good functional outcomes.
|
|
Chung et al, 2004
|
Objective: To determine tumor control, hearing preservation and toxicity rates using LINAC-based stereotactic radiation therapy.
Design: Prospective cohort, single institution experience.
Number of patients: 45 received single fraction (all functionally deaf), 27 received fractionated stereotactic radiation therapy (23 had serviceable hearing prior to radiation).
Follow-up: Median 27 months.
|
III
|
Results: Among patients receiving FRST, the 1- and 2-year hearing preservation rate was 85% and 57%, respectively.
Conclusion: Stereotactic radiotherapy provides good local tumor control and low toxicity. Fractionated treatment offers encouraging rates of hearing preservation.
|
|
Sawamura et al, 2003
|
Objective: To investigate outcomes of FRST (40–50 Gy in 20–25 fractions over 5–6 weeks) for VSs.
Design: Not specified, but assume retrospective case series, single institution experience.
Number of patients: 101 total.
Follow-up: Median 45 months.
|
III
|
Results: 78% (28/36) with serviceable hearing before radiation therapy retained serviceable hearing at last follow-up. The actuarial 5-year rate of useful hearing preservation was 71%.
Conclusion: FSRT resulted in excellent tumor control and high rates of hearing preservation. Progression to communicating hydrocephalus should be monitored closely, particularly in patients with large tumors.
|
|
Litvack et al, 2003
|
Objective: To evaluate tumor control and hearing preservation using Gamma Knife radiosurgery (12 Gy to margin) for treatment of VSs.
Design: Retrospective case series, single institution experience.
Number of patients: 134 total, 47 with serviceable hearing prior to surgery.
Follow-up: Mean 26.3 months audiometric follow-up.
|
III
|
Results: 62% (29/47) maintained serviceable hearing at a mean of 26 months following radiosurgery.
Conclusion: Patients with VSs <3 cm in maximum dimension should be given the option of radiosurgery as primary treatment.
|
|
Iwai et al, 2003
|
Objective: To report long-term outcomes following Gamma Knife radiosurgery using low dose (<12 Gy to margin) treatment.
Design: Not specified, assume retrospective, single institution experience.
Number of patients: 51 total, 18 with serviceable hearing prior to radiation.
Follow-up: Median 60 months.
|
III
|
Results: Serviceable hearing was retained in 56% (10/18) of patients with pretreatment serviceable hearing levels.
Conclusion: Low dose radiosurgery can achieve high rates of tumor control with good hearing preservation for patients with sporadic VSs
|
|
Regis et al, 2002
|
Objective: To compare outcomes following Gamma Knife radiosurgery (marginal dose of 14 Gy or less) and microsurgery for treatment of VSs.
Design: Prospective cohort, single institution experience.
Number of patients: 48 with serviceable hearing prior to radiosurgery.
Follow-up: Not specified, but reported that all patients had at least 4 years of follow-up.
|
III
|
Results: 50% of patients with serviceable pretreatment hearing maintained serviceable hearing at last follow-up. 68% of patients with GR grade I hearing before radiosurgery maintained serviceable hearing at last follow-up.
Conclusion: Findings after 4 years of follow-up indicate that radiosurgery provides better functional outcomes than microsurgery for VSs.
|
|
Petit et al, 2001
|
Objective: To evaluate tumor control and complications associated with low dose Gamma Knife radiosurgery (median 12 Gy to margin) for VSs.
Design: Not defined, assume retrospective case series, single institution experience.
Number of patients: 47 total, 26 with serviceable hearing prior to treatment.
Follow-up: Median 3.6 years.
|
III
|
Results: Hearing decreased from GR grade I to III in 3 subjects and from grade III to V in 1 patient. All patients with GR grade I or II before treatment maintained GR grade I–III at follow-up.
Conclusion: Low dose radiosurgery provides comparable tumor control and lower rates of other complications compared to prior publications.
|
|
Flickinger et al, 2001
|
Objective: To define tumor control and complications of Gamma Knife radiosurgery (median dose to margin 13 Gy) for treatment of VSs.
Design: Retrospective case series, single institution experience.
Number of patients: 190 total, 76 with serviceable hearing prior to radiation.
Follow-up: Median 30 months.
|
III
|
Results: Serviceable hearing
was preserved in 81% (61/75), with a 5-year actuarial preservation rate of 74%.
Conclusion: Radiosurgery using the current procedures is associated with a high rate of tumor control and low morbidity.
|
|
Prasad et al, 2000
|
Objective: To assess results of Gamma Knife radiosurgery (mean 13.2 Gy to margin) for treatment of VSs.
Design: Not reported, assume retrospective case series, single institution experience.
Number of patients: 153 total, 95 primary radiosurgery, 57 after prior microsurgery. 36 had serviceable hearing prior to radiosurgery.
Follow-up: Mean 4.3 years.
|
III
|
Results: 58% (21/36) of patients with serviceable pretreatment hearing maintained serviceable hearing following radiation.
Conclusion: Radiosurgery should be used to treat postoperative residual tumor and in poor surgical candidates.
|
|
Unger et al, 1999
|
Objective: To evaluate outcomes using Gamma Knife radiosurgery (12-14 Gy marginal dose) for treatment of VSs.
Design: Not reported, assume retrospective case series, single institution experience.
Number of patients: 192 total, 56 primary treatment. 46% (26/56) of patients had serviceable hearing prior to radiation.
Follow-up: Median 62 months.
|
III
|
Results: At 48 months of follow-up, 62% (16/26) of patients with serviceable hearing at time of diagnosis maintained serviceable hearing.
Conclusion: Radiosurgery provides effective treatment for VSs and is associated with an exceptionally low mortality rate and a good quality of life.
|
|
Kagei et al, 1999
|
Objective: To assess efficacy and toxicity of small field fractionated radiotherapy with or without stereotactic boost (fractionated, 44 Gy in 22 fractions often with 4 Gy boost) for treatment of VSs
Design: Not reported, assume retrospective case series, single institution experience.
Number of patients: 39 total, 15 with serviceable hearing prior to treatment.
Follow-up: Median 24 months.
|
III
|
Results: The actuarial preservation rates of serviceable hearing at 1 and 2 years were 86 and 78%, respectively.
Conclusion: Fractionated radiation with or without stereotactic boost provides good short-term tumor control and low complications when treating VSs.
|
AAO-HNS, American Academy of Otolaryngology-Head and Neck Surgery; ABR, auditory brainstem response; AHDR, annual hearing decline rate; FRST, fractionated stereotactic radiotherapy; GR, Gardner–Robertson hearing classification; IAC, internal auditory canal; LINAC, linear accelerator; VS, vestibular schwannoma.
Table 3. Surgery
|
Author/Year
|
Study Description
|
Data Class
|
Results and Conclusion
|
|
Yamakami et al, 2014
|
Objective: To report long-term functional outcomes following retrosigmoid craniotomy for resection of small (<1.5 cm) VSs.
Design: Retrospective case series, single institution experience.
Number of patients: 44 patients with AAO-HNS class A-C, 36 patients with AAO-HNS class A or B.
Follow-up: Mean 5.1 years
|
III
|
Results: 16 of 19 (84%) patients with preoperative AAO-HNS class A hearing maintained serviceable hearing following surgery. 26 of 36 (72%) of patients with AAO-HNS class A or B hearing maintained serviceable hearing following surgery. At a mean of 5.1 years, 80% of patients who had successful hearing preservation maintained AAO-HNS class A or B hearing at last follow-up.
Conclusion: Early resection of small VSs via retrosigmoid craniotomy provides cure and excellent functional outcomes.
|
|
Quist et al, 2014
|
Objective: To describe 5-year hearing preservation rates following middle fossa craniotomy for resection of VSs
Design: Retrospective case series, single institution experience.
Number of patients: 57 patients in total, 49 (86%) had preoperative serviceable hearing.
Follow-up: Not specified. Subset of patients had 5 years of follow-up that was analyzed.
|
III
|
Results: Immediate postoperative hearing was preserved in 27 (55%). 5-year follow-up data were available in 16 of 27 patients. 12 of these 16 (75%) maintained serviceable hearing at 5 years.
Conclusion: For patients who initially had hearing preserved following surgical resection of VSs, ~75% maintained serviceable hearing at 5 years.
|
|
Wang et al, 2013
|
Objective: To address hearing preservation following middle cranial fossa approach for resection of VSs. Specifically long-term durability of hearing was evaluated.
Design: Retrospective case series, single institution experience.
Number of patients: 103 total, 95 had pretreatment AAO-HNS class A or B hearing.
Follow-up: Mean 4 years.
|
III
|
Results: Following surgery, 83% (65/78) of patients with preoperative class A hearing maintained serviceable hearing, while 82% (78/95) of patients with preoperative class A or B hearing maintained serviceable hearing in the early postoperative period. Overall, a decline in AAO-HNS classification was noted in 15% of patients with preserved Class A hearing and 33% of those with preserved class B hearing.
Conclusion: Good hearing preservation and facial nerve outcomes can be achieved with the MCF approach for removal of small VSs. Durable hearing preservation is seen in most patients who initially have hearing preserved.
|
|
Vincent et al, 2012
|
Objective: To analyze impact of patient selection and intraoperative 8th nerve monitoring on hearing preservation using middle fossa craniotomy for treatment of VSs.
Design: Retrospective case series, single institution experience.
Number of patients: 77 total, 73 with pretreatment serviceable hearing.
Follow-up: Mean 8.5 years.
|
III
|
Results: Before use of auditory monitoring and excluding patients with tumors involving the cochlear fossa, hearing preservation rates following surgery were 47%. Following improved patient selection and use of 8th nerve monitoring during surgery, hearing preservation improved to 75%. The overall rate of hearing preservation for the group was 63% (36/73).
Conclusion: Use of 8th nerve monitoring and exclusion of patients with cochlear fossa enhancement results in improvement of hearing preservation following middle fossa craniotomy for resection of VSs.
|
|
Mazzoni et al, 2012
|
Objective: To evaluate long-term hearing preservation results following retrosigmoid craniotomy for resection of VSs.
Design: Retrospective case series, single institution experience.
Number of patients: 200 total, 194 with preoperative serviceable hearing.
Follow-up: Mean 14 years.
|
III
|
Results: Among all patients with preoperative serviceable hearing, overall 28% (54/189) of patients maintained serviceable hearing in the short-term and 25% (47/188) in the long-term. 44% (39/89) of patients with pretreatment class A hearing maintained serviceable hearing in the short-term following surgery, and 40% (36/89) maintained serviceable hearing in the long-term.
Conclusion: Using the retrosigmoid craniotomy for resection of VSs, 28% of patients with pretreatment serviceable hearing will maintain serviceable hearing in the short-term and 25% in the long-term. Smaller tumor size and better pretreatment hearing level predict better hearing preservation outcomes.
|
|
Hilton et al, 2011
|
Objective: To assess long-term hearing preservation following middle cranial fossa resection of VSs.
Design: Retrospective case series, single institution experience.
Number of patients: 78.
Follow-up: Mean 4 years.
|
III
|
Results: 65% (51/78) of patients with serviceable hearing before surgery maintained serviceable hearing immediately after surgery. Based on the 10-year Kaplan–Meier estimate, 72% of those who initially had hearing preserved after surgery maintained serviceable hearing.
Conclusion: Delayed hearing loss following middle fossa craniotomy for resection of VSs is uncommon. Delayed loss of serviceable hearing may indicate tumor recurrence.
|
|
Di Maio et al, 2011
|
Objective: To report the rate of hearing preservation following microsurgical resection of large (>3 cm) VSs via retrosigmoid craniotomy.
Design: Retrospective case series, single institution experience.
Number of patients: 28 all with preoperative serviceable hearing.
Follow-up: 31.3 months.
|
III
|
Results: Overall, 21% (6/28) maintained serviceable hearing following surgery. Of patients with preoperative GR grade I hearing, 38% (5/13) maintained serviceable hearing following surgery.
Conclusion: Hearing preservation is possible for patients with large tumors and should be attempted in all patients with preoperative hearing. CSF fundal fluid and less tumor extending anterior to the porus acusticus are associated with hearing preservation.
|
|
Woodson et al, 2010
|
Objective: To evaluate long-term hearing outcomes following middle fossa craniotomy for resection of VSs.
Design: Retrospective case series, single institution experience.
Number of patients: 49.
Follow-up: Mean 70.5 months.
|
III
|
Results: For subjects with >2 years of follow-up, hearing class is maintained in ~90% of patients.
Conclusion: Most patients maintain their initial postoperative hearing levels following microsurgical removal of VSs.
|
|
Sughrue et al, 2010
|
Objective: To report the functional outcome and long-term tumor control after surgery in patients <40 years of age.
Design: Retrospective case series, single institution experience.
Number of patients: 204 total, 114 with serviceable hearing who had attempted hearing preservation.
Follow-up: 10.2 years.
|
III
|
Results: The overall rate of hearing preservation for tumors <3 cm was 68% and the overall rate among tumors >3 cm was 44%. Kaplan–Meier analysis reveals that the immediate postoperative hearing test was stable over the course of follow-up.
Conclusion: Surgery provides excellent long-term tumor control and functional outcomes.
|
|
Myrseth et al, 2009
|
Objective: To compare treatment associated morbidity of radiosurgery (12 Gy marginal dose) and microsurgery for patients with VSs.
Design: Prospective, nonrandomized cohorts, single institution experience.
Number of patients: 63 radiosurgery (25 with serviceable hearing), 28 microsurgery (13 with serviceable hearing).
Follow-up: Mean 2 years.
|
II
|
Results: No patients maintained serviceable hearing at 1 and 2 years following surgery, while 76% and 68% of patients maintained serviceable hearing at 1 and 2 years following radiosurgery.
Conclusion: Better facial nerve outcomes and hearing outcomes are achieved with radiosurgery compared to microsurgery for VSs.
|
|
Gjuric et al, 2008
|
Objective: To analyze functional outcomes and to determine impact of tumor size on MCF outcomes for resection of VSs.
Design: Retrospective case series, single institution experience.
Number of patients: 197 total, 61 with serviceable hearing prior to surgery.
Follow-up: Not specified (2 months-5 years).
|
III
|
Results: Tumor size significantly predicts hearing preservation results. Specifically, the probability of hearing preservation in tumors >1.5 cm is <20%.
Conclusion: Tumor size is the primary predictor of outcome for patients undergoing MCF approach for VS resection. For facial nerve outcome, a cutoff of 0.5-cm extracanalicular extension is critical. For hearing, the probability of hearing preservation is significantly reduced in tumors >1.5 cm.
|
|
Pollock et al, 2006
|
Objective: Comparison of tumor control and functional outcomes between patients receiving microsurgery (primarily retrosigmoid approach) and radiosurgery (mean dose to margin 12.2 Gy) for VSs.
Design: Prospective, nonrandomized cohort study, single institution experience.
Number of patients: 82 total, 36 receiving microsurgery (22 with serviceable hearing before treatment), 46 receiving radiosurgery (30 with serviceable hearing before treatment).
Follow-up: Mean 42 months.
|
II
|
Results: Preservation of serviceable hearing at 1 year and last follow-up for the microsurgery cohort was 5% for both time points. Preservation of serviceable hearing at 1 year and last follow-up for radiosurgery was 63% for both time points (P < .01).
Conclusion: Early outcomes are better for radiosurgery when compared to microsurgery for <3 cm unilateral VSs.
|
|
Mohr et al, 2005
|
Objective: To examine the influence of preoperative tumor size, meatal filling and preoperative hearing levels on postoperative hearing preservation after retrosigmoid resection of VSs.
Design: Not specified, assume retrospective case series, single institution experience.
Number of patients: 128 total.
Follow-up: Not specified.
|
III
|
Results: 24% of patients maintained serviceable hearing following retrosigmoid microsurgery. Tumor size and extent of meatal filling were associated with development of nonserviceable hearing, while pretreatment hearing level was not.
Conclusion: Degree of internal auditory canal filling and tumor size are independent predictors of successful hearing preservation following microsurgery for VSs.
|
|
Lin et al, 2005
|
Objective: Comparison of hearing preservation outcomes after treatment of VSs following HFSRT (50 Gy,
25 fractions over 5 weeks), microsurgery, and observation.
Design: Retrospective case series, single institution experience.
Number of patients: HFSRT 42 (11 had serviceable hearing before radiation), microsurgery 113 all with serviceable hearing before surgery, and observation 86 (51 with serviceable hearing at diagnosis).
Follow-up: Mean follow-up HFSRT 4.0 years, microsurgery 9.5 years, and observation 6.8 years.
|
III
|
Results: 9% (1/11) maintained serviceable hearing following HFSRT, 16% (18/113) following microsurgery, and 43% (22/51) following observation.
Conclusion: Hearing decline was prevalent in all treatment groups. The decline was more significant following microsurgery and radiation compared to observation.
|
|
Grayeli et al, 2005
|
Objective: To compare conservative management with surgery for small unilateral VSs.
Design: Retrospective case series, single institution experience.
Number of patients: 44 with serviceable hearing receiving observation, 145 with serviceable hearing receiving surgery via MCF or retrosigmoid craniotomy.
Follow-up: Mean 33 months.
|
III
|
Results: Among patients who had serviceable hearing at diagnosis and received conservative management of their VSs, 57% (25/44) maintained serviceable hearing at last follow-up. Among patients undergoing hearing preservation surgery, 31% (45/145) maintained serviceable hearing. There was no difference between middle fossa and retrosigmoid resection with regard to hearing preservation success.
Conclusion: A high rate of hearing decline and loss of follow-up should be taken into consideration when evaluating hearing preservation strategies for patients with VSs.
|
|
Betchen et al, 2005
|
Objective: To determine the rate of long-term hearing preservation after retrosigmoid craniotomy for resection of VSs and to evaluate factors associated with hearing deterioration.
Design: Retrospective case series, single institution experience.
Number of patients: 142 total.
Follow-up: Mean 7 years.
|
III
|
Results: 27% (38/142) had serviceable hearing preservation in the immediate postoperative period. Of these, 85.7% maintained serviceable hearing at a mean follow-up of 7 years. The results of hearing preservation were independent of tumor size.
Conclusion: Long-term hearing preservation is maintained in 86% of patients who had hearing preserved in the immediate postoperative period. Hearing preservation is not influenced by tumor size.
|
|
Maw et al, 2003
|
Objective: To assess hearing preservation in VSs using the retrosigmoid approach.
Design: Prospective cohort, single institution experience.
Number of patients: 33 with serviceable hearing prior to surgery.
Follow-up: Median or mean not reported (range 6 months to 9 years).
|
III
|
Results: 38% of patients with serviceable hearing prior to surgery retained serviceable hearing following surgery.
Conclusion: Using appropriate surgical techniques and monitoring, it is possible to preserve serviceable hearing in approximately 50% of patients following retrosigmoid VS resection.
|
|
Friedman et al, 2003
|
Objective: To determine long-term hearing preservation following middle fossa craniotomy for resection of VSs
Design: Retrospective case series, single institution experience
Number of patients: 38 with serviceable hearing prior to surgery
Follow-up: Median or mean not reported, follow-up time up to 11 years
|
III
|
Results: 61% of patients maintained serviceable hearing immediately following surgery. 70% of these retained serviceable hearing in the 5 years following surgery.
Conclusion: More than two-thirds of patients will retain serviceable hearing at 5 years after initial successful middle fossa VS resection.
|
|
Chee et al, 2003
|
Objective: To evaluate long-term hearing preservation results following retrosigmoid craniotomy for VS resection. To identify variables associated with late audiometric decline.
Design: Retrospective case series, single institution experience
Number of patients: 126 total, 29 with serviceable hearing before surgery
Follow-up: 113.4 months
|
III
|
Results: 34% (43/126) maintained serviceable hearing immediately following surgery. 76.6% of these patients maintained serviceable hearing in the early postoperative period, and 56.7% in the late postoperative period.
Conclusion: Over time, a significant number of individuals experience greater decline in the operative ear than the non-operative ear.
|
|
Levo et al, 2002
|
Objective: To evaluate the rate and durability of hearing preservation surgery for VSs. To evaluate the perceived usefulness of preserved hearing.
Design: Not defined, assume retrospective case series, single institution experience
Number of patients: 98 with serviceable hearing prior to surgery and attempted hearing preservation
Follow-up: Mean 7.3 years
|
III
|
Results: 20.4% (20/98) hearing preservation at a mean of 7.3 years postop. Age and preoperative speech discrimination were the strongest predictors of hearing preservation.
Conclusion: Age and preoperative speech discrimination are the 2 most important predictors of hearing preservation. 66% of patients with hearing preserved rated their hearing as useful.
|
|
Lee et al, 2002
|
Objective: To evaluate the results of microsurgery for VSs utilizing the retrosigmoid approach
Design: Retrospective case series, single institution review
Number of patients: 160 total, 59 with serviceable hearing prior to surgery
Follow-up: Mean 24 months
|
III
|
Results: 19% (11/59) of patients with preoperative serviceable hearing retained serviceable hearing at last follow-up. The probability of hearing preservation was greatest in smaller tumors (25%) compared to large tumors (0%).
Conclusion: Surgical removal should be the standard management for VSs, particularly for medium and large tumors.
|
|
Kaylie et al, 2001
|
Objective: To report outcomes of VS surgery utilizing modern techniques and standardized grading. All hearing preservation attempts were via the retrosigmoid approach
Design: Retrospective case series, single institution experience
Number of patients: 97 total, 44 with serviceable hearing prior to surgery, and 37 underwent attempted hearing preservation; 27 of these had postoperative audiograms for comparison
Follow-up: Mean 49 months
|
III
|
Results: 29% (8/27) of patients with serviceable hearing maintained serviceable hearing following surgery; 29% (7/24) of small tumors, and 33% (1/3) of medium sized tumors.
Conclusion: VS surgery is safe and outcomes are good. Surgery remains the treatment of choice for most tumors.
|
|
Gjuric et al, 2001
|
Objective: To evaluate clinical outcomes following VS resection using the enlarged middle cranial fossa approach
Design: Retrospective case series, single institution experience
Number of patients: 735 total, 389 with serviceable hearing prior to surgery
Follow-up: Not reported
|
III
|
Results: 45% (176/389) with preoperative serviceable hearing retained serviceable hearing following surgery. Among patients with preoperative AAO-HNS class A hearing, 53% (135/256) retained serviceable hearing following surgery.
Conclusion: The expanded middle cranial fossa approach for VSs provides low morbidity, low risk of CSF leak, good internal auditory canal exposure and good hearing preservation for tumors <2 cm.
|
|
Kumon et al, 2000
|
Objective: To evaluate results of microsurgery for small VSs using the middle fossa and retrosigmoid approaches
Design: Retrospective case series, single institution experience
Number of patients: 53 total, 36 middle cranial fossa, 17 retrosigmoid; 40 total had serviceable hearing before surgery
Follow-up: Mean 3.75 years
|
III
|
Results: Hearing was preserved in 68% (36/53) and it was serviceable in 51% (27/53). Of patients starting with serviceable hearing, 58% (23/40) maintained serviceable hearing at last follow-up. Of patients starting with AAO-HNS class A hearing, 57% (12/21) maintained serviceable hearing at last follow-up. Hearing levels tested 1 month following surgery had not deteriorated in any patient.
Conclusion: Small (<2 cm) VSs should be surgically removed because of the high rate of hearing preservation and good facial nerve function. Tumors larger than 1 cm should be removed via retrosigmoid approach.
|
|
Ferber-Viart et al, 2000
|
Objective: To determine predictive factors of hearing preservation in patients treated with microsurgery for VSs
Design: Prospective cohort, single institution experience
Number of patients: 107 total (103 retrosigmoid, 4 middle fossa); 86 with serviceable hearing prior to surgery
Follow-up: Not reported
|
III
|
Results: 55% (47/86) of patients with preoperative serviceable hearing maintained serviceable hearing following surgery. 60% (24/40) of patients with AAO-HNS class A hearing maintained class A hearing following surgery. Tumor size, preoperative hearing levels, presence of otoacoustic emissions, short duration of hearing loss, and presence of wave III on ABR were predictors of successful hearing preservation.
Conclusion: 55% of patients with serviceable hearing will maintain serviceable hearing following surgery. Factors including ABR and OAE results, tumor size, preoperative hearing levels, and duration of hearing loss may predict hearing preservation after surgery.
|
|
Lustig et al, 1998
|
Objective: To evaluate the presentation and surgical outcome of patients with VSs who present with normal or symmetrical hearing
Design: Retrospective case series, single institution experience
Number of patients: 29, all with serviceable hearing at time of diagnosis; 21 underwent surgery, 14 retrosigmoid craniotomy, 5 middle fossa, 2 translabyrinthine
Follow-up: Not reported
|
III
|
Results: 53% (10/19) of patients undergoing attempted hearing preservation maintained serviceable hearing following surgery.
Conclusion: A small percentage of patients with VSs will present with normal audiometric findings. In this cohort, 53% maintained serviceable hearing following microsurgery with attempted hearing preservation
|
|
Kanzaki et al, 1997
|
Objective: To report outcomes following hearing preservation surgery using the middle fossa or extended middle fossa approach for VSs among patients presenting with normal hearing
Design: Not reported, assume retrospective case series, single institution experience
Number of patients: 28 with normal hearing before surgery, 53 with AAO-HNS class A, and 79 with serviceable hearing before surgery.
Follow-up: Mean 4.8 years
|
III
|
Results: Serviceable hearing was maintained in 50% (14/28) of patients presenting with normal hearing before surgery, 47% (25/53) of patients with AAO-HNS class A hearing, and 37% (29/79) of patients presenting with serviceable hearing.
Conclusion: Overall, hearing may be preserved in approximately half of patients presenting with AAO-HNS class A hearing and a third of patients receiving surgery and presenting with serviceable hearing
|
|
Gormley et al, 1997
|
Objective: To report outcomes following primarily retrosigmoid craniotomy for resection of VSs
Design: Retrospective case series, single institution experience
Number of patients: 179 total; 69 with serviceable hearing prior to surgery, 42 with <2 cm and serviceable hearing prior to surgery.
Follow-up: Median 65 months
|
III
|
Results: 48% (20/42), 25% (6/24), and 0% (0/3) of patients with <2 cm, 2–4 cm, and >4 cm tumors, respectively, and preoperative serviceable hearing maintained serviceable hearing following surgery. The overall hearing preservation rate for all patients in whom hearing preservation was attempted was 38%. None of the patients who initially had hearing preservation experienced progression to nonserviceable hearing at last follow-up.
Conclusion: Unless a patient has major medical problems, microsurgery by an experienced team of surgeons is preferred over radiosurgery. Overall, approximately 40% of patients with preoperative hearing maintain serviceable hearing following surgery. Late decline of hearing is uncommon.
|
|
Weber et al, 1996
|
Objective: To review surgical outcomes using the middle cranial fossa approach for VS resection
Design: Retrospective case series, single institution experience
Number of patients: 49, 34 with serviceable hearing prior to surgery
Follow-up: Mean 4.8 years
|
III
|
Results: Of patients with serviceable hearing prior to surgery, 50% (17/34) retained these levels after surgery.
Conclusion: 50% of patients with serviceable hearing before surgery will maintain serviceable hearing following microsurgery for small to medium-sized VSs using the middle cranial fossa approach.
|
|
Post et al, 1995
|
Objective: To report hearing preservation outcomes following retrosigmoid craniotomy for resection of VSs
Design: Not reported, assume retrospective case series, single institution experience
Number of patients: 56 total, 46 with serviceable hearing prior to surgery
Follow-up: Mean 2.5 years
|
III
|
Results: 39% (18/46) of patients with serviceable hearing prior to surgery maintained serviceable hearing after surgery. Hearing preservation rates were better with smaller tumor size.
Conclusion: Hearing preservation with retrosigmoid craniotomy is possible in 40–50% of patients. Smaller tumor size predicts increased probability of hearing preservation following surgery.
|
|
Pollock et al, 1995
|
Objective: To compare microsurgery and Gamma Knife radiosurgery (13-18 Gy marginal dose) for treatment of unilateral VSs.
Design: Retrospective case series, single institution experience
Number of patients: 87 total, microsurgery 40 (21 serviceable prior to treatment), radiosurgery 47 (8 serviceable prior to treatment).
Follow-up: Median 36 months
|
III
|
Results: At a median audiological follow-up of 35 months, 14% (3/21) of patients who received surgery, and 75% (6/8) who received radiosurgery, maintained serviceable hearing following treatment.
Conclusion: Compared to microsurgery, radiosurgery proved to be an effective and less costly management strategy for VSs <3 cm in size.
|
|
Dornhoffer et al, 1995
|
Objective: To assess hearing preservation outcomes following middle fossa for resection of VSs.
Design: Retrospective case series, single institution experience
Number of patients: 93, all with serviceable hearing
Follow-up: Not reported
|
III
|
Results: Serviceable hearing was preserved in 58% (54/93) of patients who had serviceable hearing prior to surgery. Tumor size, preoperative vertigo, and ABR findings predicted postoperative hearing preservation, while preoperative hearing levels and ENG had no prognostic value.
Conclusion: Hearing can be preserved in 58% of patients with <1.5 cm VSs using the middle fossa approach. Success rate of hearing preservation is related to tumor size.
|
|
Kanzaki et al, 1994
|
Objective: To evaluate hearing preservation rates following middle fossa and extended middle fossa craniotomy for VS resection
Design: Not reported, assume retrospective case series, single institution experience
Number of patients: 248 total, 42 with serviceable hearing and <2 cm tumor size prior to surgery
Follow-up: Not reported
|
III
|
Results: 40% (17/42) of patients with serviceable hearing and a tumor <2 cm in size retained serviceable hearing following surgery. This is compared to 1 of 4 (25%) for tumors >2 cm. Postoperative hearing deteriorated within 1 month after surgery in 3 cases. In 2 cases, hearing deteriorated during long-term postoperative follow-up because of tumor recurrence.
Conclusion: Serviceable hearing can be preserved in approximately 40% of patients after middle fossa or extended middle fossa surgery for VS resection. Hearing preservation rates are higher for smaller tumors.
|
|
Brooks et al, 1994
|
Objective: To review results of hearing preservation surgery for treatment of VSs using the retrosigmoid approach. To evaluate associations between clinical features and probability of successful hearing preservation.
Design: Not reported, assume retrospective case series, single institution experience
Number of patients: 24 total, 17 with serviceable hearing prior to surgery
Follow-up: Not reported
|
III
|
Results: 53% (9/17) of patients with preoperative serviceable hearing maintained serviceable hearing after surgery. Tumor size and tumor extension to the fundus are adverse prognostic factors for successful hearing preservation.
Conclusion: Potential hearing conservation should be considered a factor when determining best management of patients with small VSs.
|
|
Glasscock et al, 1993
|
Objective: To report the results of hearing preservation following retrosigmoid and middle fossa approaches for removal of VSs
Design: Retrospective case series, single institution experience
Number of patients: 136 total, 38 via middle fossa and 98 via retrosigmoid approach
Follow-up: mean 6.5 years
|
III
|
Results: Serviceable hearing was retained in 35% (48/136) of cases with serviceable preoperative hearing levels. Preoperative ABR results were useful in predicting outcome of hearing preservation surgery.
Conclusion: Serviceable hearing can be maintained in 35% of patients who present with serviceable hearing.
|
|
Goel et al, 1992
|
Objective: To report the late course of hearing preservation and tinnitus following retrosigmoid craniotomy for VSs.
Design: Retrospective case series, single institution experience
Number of patients: 42
Follow-up: Median 2.5 years
|
III
|
Results: 15 of 42 (36%) patients selected for hearing preservation attempt had GR grade I-III following surgery at a median follow-up of 2.5 years. Thirteen of 42 (31%) maintained serviceable hearing (GR grade I or II). Hearing preservation outcomes were better in patients with smaller tumors.
Conclusion: Smaller tumor size is associated with better hearing preservation rates. Delayed hearing loss may occur in patients who initially have hearing preserved following VS surgery. A fraction of patients may experience hearing improvement following surgery.
|
|
Fischer et al, 1992
|
Objective: To report hearing preservation results following retrosigmoid craniotomy for resection of VSs and to identify predictors of outcome
Design: Retrospective case series, single institution experience
Number of patients: 99
Follow-up: Mean 5.2 years
|
III
|
Results: 22 patients had serviceable hearing before surgery and 12 (55%) maintained serviceable hearing following surgery at a median follow-up of 5.5 years. Tumor size, preoperative pure tone levels, and use of BAER were associated with better hearing preservation outcomes.
Conclusion: Smaller tumor size and preoperative pure tone thresholds predict hearing preservation outcome. Use of BAER is associated with higher rates of hearing preservation.
|
AAO-HNS, American Academy of Otolaryngology-Head and Neck Surgery; ABR, auditory brainstem response; BAER, brainstem auditory evoked response; CSF, cerebrospinal fluid; ENG, electronystamography; GR, Gardner–Robertson hearing classification; HSRT, hypofractionated stereotactic radiotherapy; OAE, otoacoustic emissions; VS, vestibular schwannoma.
Table 4. Observation
|
Author/Year
|
Study Description
|
Data Class
|
Results and Conclusion
|
|
Fayad et al, 2014
|
Objective: To evaluate long-term tumor control and hearing preservation among conservatively managed VSs
Design: Retrospective case series, single institution experience
Number of patients: 114 total patients, 32 with serviceable hearing at presentation
Follow-up: Mean 4.8 years radiologic, mean 6.4 years any type of follow-up
|
III
|
Results: Of patients presenting with serviceable hearing, 59% (19/32) maintained serviceable hearing. Of patients with AAO-HNS Class A hearing at presentation, 86% (12/14) maintained serviceable hearing.
Conclusion: Of patients electing initial observation, approximately 31% may eventually undergo further treatment.
|
|
Breivik et al, 2013
|
Objective: To evaluate the effect of Gamma Knife radiosurgery on growth and hearing compared to conservatively managed VSs with extracanalicular extension
Design: Prospective cohort study, single institution experience
Number of patients: 237 total; 113 receiving radiosurgery, 124 conservatively managed. 114 patients had serviceable hearing prior to radiosurgery.
Follow-up: Mean 55 months
|
II
|
Results: Serviceable hearing was lost in 76% (54/71) of patients with observed tumors and 64% (34/53) who received radiosurgery (not a statistically significant difference).
Conclusion: Gamma Knife radiosurgery reduces the tumor growth rate compared to conservatively managed tumors. Hearing is lost at a similar rate between groups. Symptoms and quality of life are not different between groups.
|
|
Sughrue et al, 2011
|
Objective: To evaluate the natural history of hearing loss in a cohort of patients with conservatively managed VSs
Design: Prospective cohort study, single institution experience
Number of patients: 59 total, all with serviceable hearing at diagnosis
Follow-up: Mean 5.3 years
|
II
|
Results: The estimated median time to non-serviceable hearing ranged from 9.3-11.6 years for the three different tumor size groups. Growth rate (2.5mm/yr cut-point) was the strongest predictor of hearing loss. Initial tumor size and age did not affect time to serviceable hearing.
Conclusion: Rapid tumor growth portends hearing loss. More than half of patients at 10 years, and more than 80% of patients at 20 years will acquire nonserviceable hearing during the course of conservative observation.
|
|
Pennings et al, 2011
|
Objective: To evaluate the natural course of hearing loss during conservative observation of intracanalicular VSs
Design: Retrospective case series, single institution experience
Number of patients: 47 total, 31 with serviceable hearing at diagnosis
Follow-up: Mean 3.6 years
|
III
|
Results: 74% of subjects with serviceable hearing at time of diagnosis maintained serviceable hearing during the course of observation. Growth status or tumor location did not predict loss of serviceable hearing.
Conclusion: Hearing will deteriorate in a percentage of patients with observed VSs, regardless of tumor growth. Hearing loss commonly occurs at the early part of observation.
|
|
Stangerup et al, 2010
|
Objective: Evaluate long-term hearing during “wait and scan” management of VSs
Design: Retrospective case series, single institution experience
Number of patients: 932 total, 455 with serviceable hearing at diagnosis
Follow-up: Median or mean not specified
|
III
|
Results: 51% of patients with AAO-HNS class A hearing at diagnosis maintained class A hearing after the observation period. 81% of patients with AAO-HNS class A hearing at diagnosis maintained serviceable hearing at last follow-up. 55% of patients with serviceable hearing at time of diagnosis maintained serviceable hearing at last follow-up.
Conclusion: Most patients with VSs presenting with 100% speech discrimination at diagnosis maintain good hearing after many years of observation.
|
|
Regis et al, 2010
|
Objective: To compare tumor control and hearing outcomes between patients receiving conservative management and upfront radiosurgery (marginal dose 12 Gy) for treatment of VSs
Design: Prospective cohort study, single institution experience
Number of patients: 47 receiving observation (31 with serviceable hearing) and 34 receiving radiosurgery, all had functional hearing
Follow-up: Median 34.7 months
|
II
|
Results: Serviceable hearing preservation in the observation group at 3, 4, and 5 years was 75%,52%, and 41%, respectively. Serviceable hearing preservation in the radiosurgery group at 3, 4, and 5 years was 77%, 70%, and 64%, respectively
Conclusion: Conservative treatment is associated with an increased risk of tumor growth and loss of serviceable hearing compared to upfront radiosurgery
|
|
Stangerup et al, 2008
|
Objective: To evaluate hearing changes during observation of VSs
Design: Retrospective case series, single institution experience
Number of patients: 314 patients with serviceable hearing at diagnosis
Follow-up: Mean 4.0 years
|
III
|
Results: For patients with AAO-HNS class A hearing at diagnosis, 74.4% maintained serviceable hearing at last follow-up. For patients with AAO-HNS class A or B hearing at diagnosis, 49% maintained serviceable hearing at last follow-up.
Conclusion: After comparing hearing outcomes between microsurgery, radiation therapy, and observation, it appears that the main indication for treatment should be tumor growth and not proactive treatment for hearing preservation.
|
|
Ferri et al, 2008
|
Objective: To evaluate outcomes of conservative management for VSs
Design: Prospective cohort study, single institution experience
Number of patients: 123 total, 56 with serviceable hearing at diagnosis
Follow-up: Mean 4.8 years
|
III
|
Results: During the course of observation, 73% (41/56) of patients maintained serviceable hearing at last follow-up regardless of tumor growth.
Conclusion: Conservative management of VSs appears safe since most tumors do not grow, and surgical outcomes are not affected by possible delays. In most cases, useful hearing is maintained over time.
|
|
Quaranta et al, 2007
|
Objective: To evaluate change in hearing and tinnitus in a cohort of patients with unilateral VSs who were initially managed with conservative observation
Design: Retrospective case series, single institution experience
Number of patients: 70 total, 15 with serviceable hearing at diagnosis
Follow-up: Mean 33 months
|
III
|
Results: 60% (9/15) of patients with serviceable hearing at diagnosis maintained serviceable hearing at last follow-up. Growth and tinnitus predicted hearing deterioration.
Conclusion: The risk of losing eligibility for hearing preservation surgery was less than 30% after a mean follow-up of 33.3 months.
|
|
Caye-Thomasen et al, 2007
|
Objective: To report hearing preservation outcomes among patients with intracanalicular VSs managed with observation
Design: Retrospective case series, single institution experience
Number of patients: 156 total, 70 with serviceable hearing at diagnosis
Follow-up: Mean 4.6 years
|
III
|
Results: The risk of significant hearing loss was 54% during 4.6 years of observation. Loss of pure tone average was smaller in shrinking tumors, and the rate of loss was higher in growing tumors.
Conclusion: Volumetric growth is associated with hearing loss. The proportion of patients eligible for hearing preservation treatment was reduced to 28% during the course of observation.
|
|
Lin et al, 2005
|
Objective: Comparison of hearing preservation outcomes after treatment of VSs following hyperfractionated stereotactic radiosurgery (50 Gy total in
25 fractions over 5 weeks), microsurgery, and observation.
Design: Retrospective case series, single institution experience
Number of patients: HFSRT 42 (11 had serviceable hearing before radiation), microsurgery 113, all with serviceable hearing before surgery, and observation, 86 (51 with serviceable hearing at diagnosis).
Follow-up: Mean follow-up HFSRT 4.0 years, microsurgery 9.5 years, and observation 6.8 years
|
III
|
Results: 9% (1/11) maintained serviceable hearing following HFSRT, 16% (18/113) following microsurgery, and 43% (22/51) following observation.
Conclusion: Hearing decline was prevalent in all treatment groups. The decline was most significant following microsurgery and radiation compared to observation.
|
|
Grayeli et al, 2005
|
Objective: To compare conservative management with surgery for small unilateral VSs
Design: Retrospective case series, single institution experience
Number of patients: 44 with serviceable hearing receiving observation, 145 with serviceable hearing receiving surgery via middle cranial fossa or retrosigmoid craniotomy
Follow-up: Mean 33 months
|
III
|
Results: Among patients who had serviceable hearing at diagnosis and received conservative management of their VSs, 57% (25/44) maintained serviceable hearing at last follow-up. Among patients undergoing hearing preservation surgery, 31% (45/145) maintained serviceable hearing. There was no difference between middle fossa and retrosigmoid resection with regard to hearing preservation.
Conclusion: A high rate of hearing loss and loss of patient follow-up should be taken into consideration when evaluating hearing preservation strategies for patients with VSs.
|
|
Walsh et al, 2000
|
Objective: To determine the risk of hearing loss during conservative observation of VSs
Design: Retrospective case series, single institution experience
Number of patients: 25, 12 with serviceable hearing at diagnosis
Follow-up: Mean 44 months
|
III
|
Results: 58% (7/12) of patients with serviceable hearing at diagnosis maintained serviceable hearing at last follow-up. 57% (4/7) with AAO-HNS class A hearing at diagnosis retained serviceable hearing at last follow-up.
Conclusion: There is significant risk to lose serviceable hearing during conservative management of VSs. The risk is greatest in tumors demonstrating growth.
|
|
Massick et al, 2000
|
Objective: To prospectively evaluate correlation between tumor volume, growth, and hearing change in conservatively managed VSs
Design: Prospective cohort, single institution experience
Number of patients: 21 total, 14 with serviceable hearing at diagnosis, 8 non-NF2 with serviceable hearing at diagnosis
Follow-up: Mean 3.8 years
|
III
|
Results: There is a significant correlation between change in tumor volume and changes in pure tone average and speech discrimination score. Of non-NF2 patients presenting with serviceable hearing, 50% maintained serviceable hearing after a mean of 4 years of follow-up.
Conclusion: Volumetric growth predicts hearing deterioration during conservative management of VSs.
|
|
Charabi et al, 1995
|
Objective: Evaluate consequences of the “wait-and-see” approach to VS management
Design: Prospective cohort, multicenter study
Number of patients: 123 total, 37 with serviceable hearing at time of diagnosis
Follow-up: Mean 3.4 years
|
III
|
Results: During the course of observation, 62% (23/37) developed nonserviceable hearing.
Conclusion: Growth was observed in 74%, and loss of serviceable hearing was seen in 62% of patients during conservative management of VSs.
|
AAO-HNS, American Academy of Otolaryngology-Head and Neck Surgery; NF2, neurofibromatosis 2; VS, vestibular schwannoma.
References
1. Ramsden RT. The bloody angle: 100 years of acoustic neuroma surgery. J R Soc Med 1995;88(8):464P-468P.
2. Akard W, Tubbs RS, Seymour ZA, Hitselberger WE, Cohen-Gadol AA. Evolution of techniques for the resection of vestibular schwannomas: from saving life to saving function. J Neurosurg 2009;110(4):642-647.
3. Cushing H. Tumors of the Nervus Acusticus and the Syndrome of the Cerbellopontile Angle. Philadelphia (PA): W.B. Saunders; 1917.
4. Dandy WE. Results of removal of acoustic tumors by unilateral approach. Arch Surg 1941;42:1026-1033.
5. House WF. Transtemporal bone microsurgical removal of acoustic neuromas. Evolution of transtemporal bone removal of acoustic tumors. Arch Otolaryngol 1964;80:731-742.
6. Pulec JL, House WF. Transtemporal bone microsurgical removal of acoustic neuromas. Facial nerve involvement and testing in acoustic neuromas. Arch Otolaryngol 1964;80:685-692.
7. House HP, House WF. Transtemporal Bone microsurgical removal of acoustic neuromas. historical review and problem of acoustic neuroma. Arch Otolaryngol 1964;80:601-604.
8. House WF. Transtemporal bone microsurgical removal of acoustic neuromas. Report of cases. Arch Otolaryngol 1964;80:617-667.
9. House WF. Surgical exposure of the internal auditory canal and its contents through the middle, cranial fossa. Laryngoscope 1961;71:1363-1385.
10. Carlson ML, Link MJ, Wanna GB, Driscoll CL. Management of sporadic vestibular schwannoma. Otolaryngol Clin North Am 2015;48(3):407-422.
11. Ganz JC. Stockholm radiosurgery developing 1968-1982. Prog Brain Res 2014;215:85-94.
12. Lasak JM, Gorecki JP. The history of stereotactic radiosurgery and radiotherapy. Otolaryngol Clin North Am 2009;42(4):593-599.
13. Leksell L. A note on the treatment of acoustic tumours. Acta Chir Scand 1971;137(8):763-765.
14. Delgado TE, Bucheit WA, Rosenholtz HR, Chrissian S. Intraoperative monitoring of facila muscle evoked responses obtained by intracranial stimulation of the facila nerve: a more accurate technique for facila nerve dissection. Neurosurgery 1979;4(5):418-421.
15. Roberson J, Senne A, Brackmann D, Hitselberger WE, Saunders J. Direct cochlear nerve action potentials as an aid to hearing preservation in middle fossa acoustic neuroma resection. Am J Otol1996;17(4):653-657.
16. Strasnick B, Glasscock 3rd ME, Haynes D, McMenomey SO, Minor LB. The natural history of untreated acoustic neuromas. Laryngoscope 1994;104(9):1115-1119.
17. Carlson ML, Habermann EB, Wagie AE, et al. The changing landscape of vestibular schwannoma management in the United States—a shift toward conservatism. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2015;153(3):440-446.
18. Stangerup SE, Caye-Thomasen P. Epidemiology and natural history of vestibular schwannomas. Otolaryng Clin N Am 2012;45(2):257-+.
19. Stangerup SE, Thomsen J, Tos M, Caye-Thomasen P. Long-term hearing preservation in vestibular schwannoma. Otol Neurotol 2010;31(2):271-275.
20. Hanson H. Unilateral deafness a s, psychological, and existential aspects [thesis]. Stockholm, Sweden: University of Stolkholm, 1993.
21. Wie OB, Pripp AH, Tvete O. Unilateral deafness in adults: effects on communication and social interaction. Ann Otol Rhinol Laryngol 2010;119(11):772-781.
22. Tveiten OV, Carlson ML, Goplen F, Vassbotn F, Link MJ, Lund-Johansen M. Long-term Auditory symptoms in patients with sporadic vestibular schwannoma: an international cross-sectional study. Neurosurgery 2015;77(2):218-227.
23. Han SJ, Oh MC, Sughrue ME, et al. The effect of the 2003 Consensus Reporting Standards on publications describing patients with vestibular schwannoma treated with stereotactic radiosurgery. J Clin Neurosci 2012;19(8):1144-1147.
24. Han SJ, Oh MC, Sughrue ME, Aranda D, Rutkowski MJ, Parsa AT. Reporting standard compliance in publications of vestibular schwannoma patients treated with microsurgery. Otol Neurotol 2012;33(4):648-650.
25. Bassim MK, Berliner KI, Fisher LM, Brackmann DE, Friedman RA. Radiation therapy for the treatment of vestibular schwannoma: a critical evaluation of the state of the literature. Otol Neurotol 2010;31(4):567-573.
26. Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC. Otolaryngol Head Neck Surg 1995;113(3):179-180.
27. Kanzaki J, Tos M, Sanna M, Moffat DA, Monsell EM, Berliner KI. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol 2003;24(4):642-648.
28. Gurgel RK, Jackler RK, Dobie RA, Popelka GR. A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol Head Neck Surg 2012;147(5):803-807.
29. Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol 1988;97(1):55-66.
30. Woodson EA, Dempewolf RD, Gubbels SP, et al. Long-term hearing preservation after microsurgical excision of vestibular schwannoma. Otol Neurotol 2010;31(7):1144-1152.
31. Shelton C, Brackmann DE, House WF, Hitselberger WE. Acoustic tumor surgery. Prognostic factors in hearing conversation. Arch Otolaryngol Head Neck Surg 1989;115(10):1213-1216.
32. Jannetta PJ, Moller AR, Moller MB. Technique of hearing preservation in small acoustic neuromas. Ann Surg 1984;200(4):513-523.
33. Silverstein H, McDaniel A, Norrell H, Haberkamp T. Hearing preservation after acoustic neuroma surgery with intraoperative direct eighth cranial nerve monitoring: Part II. A classification of results. Otolaryngol Head Neck Surg 1986;95(3 part 1):285-291.
34. Samii M, Gerganov V, Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg 2006;105(4):527-535.
35. Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): hearing function in 1000 tumor resections. Neurosurgery 1997;40(2):248-260.
36. Niranjan A, Lunsford LD, Flickinger JC, Maitz A, Kondziolka D. Dose reduction improves hearing preservation rates after intracanalicular acoustic tumor radiosurgery. Neurosurgery 1999;45(4):753-762.
37. Chopra R, Kondziolka D, Niranjan A, Lunsford LD, Flickinger JC. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Physics 2007;68(3):845-851.
38. Kastner M, Wilczynski NL, Walker-Dilks C, McKibbon KA, Haynes B. Age-specific search strategies for Medline. J Med Internet Res 2006;8(4):e25.
39. Haynes RB, McKibbon KA, Wilczynski NL, Walter SD, Werre SR, Hedges T. Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. BMJ (Clinical research ed.) 2005;330(7501):1179.
40. Montori VM, Wilczynski NL, Morgan D, Haynes RB, Hedges T. Optimal search strategies for retrieving systematic reviews from Medline: analytical survey. BMJ Clin Res Ed 2005;330(7482):68.
41. Wong SS, Wilczynski NL, Haynes RB. Comparison of top-performing search strategies for detecting clinically sound treatment studies and systematic reviews in MEDLINE and EMBASE. J Med Libr Assoc 2006;94(4):451-455.
42. Zhang L, Ajiferuke I, Sampson M. Optimizing search strategies to identify randomized controlled trials in MEDLINE. BMC Med Res Methodol 2006;6:23.
43. Topfer LA, Parada A, Menon D, Noorani H, Perras C, Serra-Prat M. Comparison of literature searches on quality and costs for health technology assessment using the MEDLINE and EMBASE databases. Int J Technol Assess Health Care 1999;15(2):297-303.
44. Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound prognostic studies in MEDLINE: an analytic survey. BMC Med 2004;2:23.
45. Wilczynski NL, Haynes RB, Hedges T. EMBASE search strategies achieved high sensitivity and specificity for retrieving methodologically sound systematic reviews. J Clin Epidemiol 2007;60(1):29-33.
46. Puataweepong P, Dhanachai M, Dangprasert S, et al. Linac-based stereotactic radiosurgery and fractionated stereotactic radiotherapy for vestibular schwannomas: comparative observations of 139 patients treated at a single institution. J Radiat Res 2014;55(2):351-358.
47. Kranzinger M, Zehentmayr F, Fastner G, et al. Hypofractionated stereotactic radiotherapy of acoustic neuroma. Strahlenther Onkol 2014;190(9):798-805.
48. Jacob JT, Carlson ML, Schiefer T, Pollock B, Driscoll C, Link M. Significance of cochlear dose in the radiosurgical treatment of vestibular schwannoma: controversies and unanswered questions. Neurosurgery 2014;74(5):466-474.
49. Tsai JT, Lin JW, Lin CM, et al. Clinical evaluation of CyberKnife in the treatment of vestibular schwannomas. Biomed Res Int 2013;2013:297093.
50. Vivas EX, Wegner R, Conley G, et al. Treatment outcomes in patients treated with CyberKnife radiosurgery for vestibular schwannoma. Otol Neurotol 2014;35(1):162-170.
51. Massager N, Nissim O, Delbrouck C, et al. Irradiation of cochlear structures during vestibular schwannoma radiosurgery and associated hearing outcome. J Neurosurg 2013;119:82-88.
52. Lunsford LD, Niranjan A, Flickinger JC, Maitz A, Kondziolka D. Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg 2013;119:24-28.
53. Litre F, Rouzzeaux P, Jovenin N, et al. Fractionated stereotactic radiotherapy for acoustic neuromas: a prospective monocenter study of about 158 cases. Radiother Oncol 2013;106(2):169-174.
54. Kim YH, Kim DG, Han JH, et al. Hearing outcomes after stereotactic radiosurgery for unilateral intracanalicular vestibular schwannomas: implication of transient volume expansion. Int J Radiat Oncol Biol Phys 2013;85(1):61-67.
55. Hayden Gephart MG, Hansasuta A, Balise RR, et al. Cochlea radiation dose correlates with hearing loss after stereotactic radiosurgery of vestibular schwannoma. World Neurosurg 2013;80(3-4):359-363.
56. Combs SE, Welzel T, Kessel K, et al. Hearing preservation after radiotherapy for vestibular schwannomas is comparable to hearing deterioration in healthy adults and is accompanied by local tumor control and a highly preserved quality of life (QOL) as patients’ self-reported outcome. Radiother Oncol 2013;106(2):175-180.
57. Champ CE, Shen X, Shi W, et al. Reduced-dose fractionated stereotactic radiotherapy for acoustic neuromas: maintenance of tumor control with improved hearing preservation. Neurosurgery 2013;73(3):489-496.
58. Carlson ML, Jacob JT, Driscoll CL, et al. Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma. J Neurol Surg Part B Skull Base 2012;73:A253.
59. Baschnagel AM, Chen PY, Bojrab D, et al. Hearing preservation in patients with vestibular schwannoma treated with Gamma Knife surgery. J Neurosurg 2013;118(3):571-578.
60. Yomo S, Carron R, Thomassin JM, Roche PH, Regis J. Longitudinal analysis of hearing before and after radiosurgery for vestibular schwannoma. J Neurosurg 2012;117(5):877-885.
61. Sun S, Liu A. Long-term follow-up studies of Gamma Knife surgery with a low margin dose for vestibular schwannoma. J Neurosurg 2012;117(suppl):57-62.
62. Rasmussen R, Claesson M, Stangerup SE, et al. Fractionated stereotactic radiotherapy of vestibular schwannomas accelerates hearing loss. Int J Radiat Oncol 2012;83(5):E607-E611.
63. Han JH, Kim DG, Chung HT, et al. Hearing preservation in patients with unilateral vestibular schwannoma who undergo stereotactic radiosurgery. Cancer 2012;118(21):5441-5447.
64. Kopp C, Fauser C, Müller A, et al. Stereotactic fractionated radiotherapy and linac radiosurgery in the treatment of vestibular schwannoma-report about both stereotactic methods from a single institution. Int J Radiat Oncol 2011;80(5):1485-1491.
65. Kim JW, Kim DG, Paek S, et al. Efficacy of corticosteroids in hearing preservation after radiosurgery for vestibular schwannoma: a prospective study. Stereot Funct Neurosurg 2011;89(1):25-33.
66. Hasegawa T, Kida Y, Kato T, Iizuka H, Yamamoto T. Factors associated with hearing preservation after Gamma Knife surgery for vestibular schwannomas in patients who retain serviceable hearing. J Neurosurg 2011;115(6):1078-1086.
67. Hansasuta A, Choi CY, Gibbs IC, et al. Multisession stereotactic radiosurgery for vestibular schwannomas: single-institution experience with 383 cases. Neurosurgery 2011;69(6):1200-1209.
68. Collen C, Ampe B, Gevaert T, et al. Single fraction versus fractionated linac-based stereotactic radiotherapy for vestibular schwannoma: a single-institution experience. Int J Radiat Oncol 2011;81(4):E503-E509.
69. Régis J, Carron R, Park MC, et al. Wait-and-see strategy compared with proactive Gamma Knife surgery in patients with intracanalicular vestibular schwannomas. J Neurosurg 2010;113(suppl):105-111.
70. Tamura M, Carron R, Yomo S, et al. Hearing preservation after Gamma Knife radiosurgery for vestibular schwannomas presenting with high-level hearing. Neurosurgery 2009;64(2):289-296.
71. Myrseth E, Møller P, Pedersen PH, Lund-Johansen M. Vestibular schwannoma: surgery or Gamma Knife radiosurgery? A prospective, nonrandomized study. Neurosurgery 2009;64(4):654-661.
72. Lasak JK, D, Kryzer, T, Hearn, C, Gorecki, J, Rine, G. Gamma knife radiosurgery for vestibular schwannoma: early hearing outcomes and evaluation of the cochlear dose. Otol Neurotol 2008;29(8):1179-1186.
73. Thomas C, Di Maio S, Ma R, et al. Hearing preservation following fractionated stereotactic radiotherapy for vestibular schwannomas: prognostic implications of cochlear dose. J Neurosurg 2007;107(5):917-926.
74. Chopra R, Kondziolka D, Niranjan A, Lunsford LD, Flickinger JC. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol 2007;68(3):845-851.
75. Pollock BE, Dirscoll CL, Foote RL, et al. Patient outcomes after vestibular schwannoma management: A prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery 2006;59(1):77-83.
76. Massager N, Nissim O, Delbrouck C, et al. Role of intracanalicular volumetric and dosimetric parameters on hearing preservation after vestibular schwannoma radiosurgery. Int J Radiat Oncol 2006;64(5):1331-1340.
77. Paek SH, Chung HT, Jeong SS, et al. Hearing preservation after gamma knife stereotactic radiosurgery of vestibular schwannoma. Cancer 2005;104(3):580-590.
78. Hasegawa T, Fujitani S, Katsumata S, Kida Y, Yoshimoto M, Koike J. Stereotactic radiosurgery for vestibular schwannomas: analysis of 317 patients followed more than 5 years. Neurosurgery 2005;57(2):257-263.
79. Combs SE, Thilmann C, Debus J, Schulz-Ertner D. Long-term outcome of stereotactic radiosurgery (SRS) in patients with acoustic neuromas. Int J Radiat Oncol 2006;64(5):1341-1347.
80. Combs SE, Volk S, Schulz-Ertner D, Huber PE, Thilmann C, Debus J. Management of acoustic neuromas with fractionated stereotactic radiotherapy (FSRT): long-term results in 106 patients treated in a single institution. Int J Radiat Oncol 2005;63(1):75-81.
81. Flickinger JC, Kondziolka D, Niranjan A, Maitz A, Voynov G, Lunsford LD. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol 2004;60(1):225-230.
82. Chung HT, Ma R, Toyota B, Clark B, Robar J, McKenzie M. Audiologic and treatment outcomes after linear accelerator-based stereotactic irradiation for acoustic neuroma. Int J Radiat Oncol 2004;59(4):1116-1121.
83. Sawamura Y, Shirato H, Sakamoto T, et al. Management of vestibular schwannoma by fractionated stereotactic radiotherapy and associated cerebrospinal fluid malabsorption. J Neurosurg 2003;99(4):685-692.
84. Litvack ZN, Norén G, Chougule PB, Zheng Z. Preservation of functional hearing after gamma knife surgery for vestibular schwannoma. Neurosurg Focus 2003;14(5):e3.
85. Iwai Y, Yamanaka K, Shiotani M, Uyama T. Radiosurgery for acoustic neuromas: results of low-dose treatment. Neurosurgery 2003;53(2):282-287.
86. Régis J, Pellet W, Delsanti C, et al. Functional outcome after gamma knife surgery or microsurgery for vestibular schwannomas. J Neurosurg 2002;97(5):1091-1100.
87. Petit JH, Hudes RS, Chen TT, Eisenberg HM, Simard JM, Chin LS. Reduced-dose radiosurgery for vestibular schwannomas. Neurosurgery 2001;49(6):1299-1306.
88. Flickinger JC, Kondziolka D, Niranjan A, Lunsford LD. Results of acoustic neuroma radiosurgery: an analysis of 5 years’ experience using current methods. J Neurosurg 2001;94(1):1-6.
89. Prasad D, Steiner M, Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg 2000;92(5):745-759.
90. Unger F, Walch C, Haselsberger K, et al. Radiosurgery of vestibular schwannomas: a minimally invasive alternative to microsurgery. Acta Neurochir 1999;141(12):1281-1286.
91. Kagei K, Shirato H, Suzuki K, et al. Small-field fractionated radiotherapy with or without stereotactic boost for vestibular schwannoma. Radiother Oncol 1999;50(3):341-347.
92. Breivik CN, Nilsen RM, Myrseth E, et al. Conservative management or gamma knife radiosurgery for vestibular schwannoma: tumor growth, symptoms, and quality of life. Neurosurgery 2013;73(1):48-56.
93. Coucke P, Janvary ZL, Baart V, Devillers M, Jansen N, Lenaerts E. I beg your pardon? I can be treated by another modality than surgery for my vestibular schwannoma [in French]. Rev Med Liege 2011;66(10):521-528.
94. Regis J, Carron R, Park MC, et al. Wait-and-see strategy compared with proactive Gamma Knife surgery in patients with intracanalicular vestibular schwannomas. J Neurosurg 2013;119 Suppl:105-111.
95. Brackmann DE, Fayad JN, Slattery 3rd WH, et al. Early proactive management of vestibular schwannomas in neurofibromatosis type 2. Neurosurgery 2001;49(2):274-280.
96. Meyer TA, Canty PA, Wilkinson EP, Hansen MR, Rubinstein JT, Gantz BJ. Small acoustic neuromas: surgical outcomes versus observation or radiation. Otol Neurotol 2006;27(3):380-392.
97. Stangerup SE, Thomsen J, Tos M, Caye-Thomasen P. Long-term hearing preservation in vestibular schwannoma. Otol Neurotol 2010;31(2):271-275.
98. Yates PD, Jackler RK, Satar B, Pitts LH, Oghalai JS. Is it worthwhile to attempt hearing preservation in larger acoustic neuromas? Otol Neurotol 2003;24(3):460-464.
99. Carlson ML, Jacob JT, Pollock BE, et al. Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: patterns of hearing loss and variables influencing audiometric decline. J Neurosurg 2013;118(3):579-587.
100. Milligan BD, Pollock BE, Foote RL, Link MJ. Long-term tumor control and cranial nerve outcomes following gamma knife surgery for larger-volume vestibular schwannomas. J Neurosurg 2012;116(3):598-604.
101. Lin VY, Stewart C, Grebenyuk J, et al. Unilateral acoustic neuromas: long-term hearing results in patients managed with fractionated stereotactic radiotherapy, hearing preservation surgery, and expectantly. Laryngoscope 2005;115(2):292-296.
102. Yang I, Sughrue ME, Han SJ, et al. A comprehensive analysis of hearing preservation after radiosurgery for vestibular schwannoma. J Neurosurg 2010;112(4):851-859.
103. Yamakami I, Ito S, Higuchi Y. Retrosigmoid removal of small acoustic neuroma: curative tumor removal with preservation of function. J Neurosurg 2014;121(3):554-563.
104. Quist TS, Givens DJ, Gurgel R, Chamoun R, Shelton C. Hearing preservation after middle fossa vestibular schwannoma removal: are the results durable? Otolaryngol Head Neck Surg 2015;152(4):706-711.
105. Wang AC, Chinn SB, Than KD, et al. Durability of hearing preservation after microsurgical treatment of vestibular schwannoma using the middle cranial fossa approach. J Neurosurg 2013;119(1):131-138.
106. Vincent C, Bonne NX, Guerin C, et al. Middle fossa approach for resection of vestibular schwannoma: impact of cochlear fossa extension and auditory monitoring on hearing preservation. Otol Neurotol 2012;33(5):849-852.
107. Mazzoni A, Zanoletti E, Calabrese V. Hearing preservation surgery in acoustic neuroma: long-term results. Acta Otorhinolaryngol Ital 2012;32(2):98-102.
108. Hilton CW, Haines SJ, Agrawal A, Levine SC. Late failure rate of hearing preservation after middle fossa approach for resection of vestibular schwannoma. Otol Neurotol 2011;32(1):132-135.
109. Di Maio S, Malebranche AD, Westerberg B, Akagami R. Hearing preservation after microsurgical resection of large vestibular schwannomas. Neurosurgery 2011;68(3):632-640.
110. Woodson EA, Dempewolf RD, Gubbels SP, et al. Long-term hearing preservation after microsurgical excision of vestibular schwannoma. Otol Neurotol 2010;31(7):1144-1152.
111. Sughrue ME, Kaur R, Rutkowski M, et al. A critical evaluation of vestibular schwannoma surgery for patients younger than 40 years of age. Neurosurgery 2010;67(6):1646-1653.
112. Gjuric M, Rudic M. What is the best tumor size to achieve optimal functional results in vestibular schwannoma surgery? Skull Base 2008;18(5):317-325.
113. Mohr G, Sade B, Dufour JJ, Rappaport J. Preservation of hearing in patients undergoing microsurgery for vestibular schwannoma: degree of meatal filling. J Neurosurg 2005;102(1):1-5.
114. Lin VY, Stewart C, Grebenyuk J, et al. Unilateral acoustic neuromas: long-term hearing results in patients managed with fractionated stereotactic radiotherapy, hearing preservation surgery, and expectantly. Laryngoscope 2005;115(2):292-296.
115. Bozorg Grayeli A, Kalamarides M, Ferrary E, et al. Conservative management versus surgery for small vestibular schwannomas. Acta Otolaryngol 2005;125(10):1063-1068.
116. Betchen SA, Walsh J, Post KD. Long-term hearing preservation after surgery for vestibular schwannoma. J Neurosurg 2005;102(1):6-9.
117. Maw AR, Coakham HB, Ayoub O, Butler SR. Hearing preservation and facial nerve function in vestibular schwannoma surgery. Clin Otolaryngol 2003;28(3):252-256.
118. Friedman RS, Kesser B, Brackmann D, Fisher L, Slattery W, Hitselberger W. Long-term hearing preservation after middle fossa removal of vestibular schwannoma. Otolaryngol Head Neck Surg 2003;129(6):660-665.
119. Chee GH, Nedzelski JM, Rowed D. Acoustic neuroma surgery: the results of long-term hearing preservation. Otol Neurotol 2003;24(4):672-676.
120. Levo H, Blomstedt G, Pyykko I. Is hearing preservation worthwhile in vestibular schwannoma surgery? Acta Otolaryngol 2000:26-27.
121. Lee SH, Wilcox TO, Buchheit WA. Current results of the surgical management of acoustic neuroma. Skull Base 2002;12(4):189-196.
122. Kaylie DM, Gilbert E, Horgan MA, Delashaw JB, McMenomey SO. Acoustic neuroma surgery outcomes. Otol Neurotol 2001;22(5):686-689.
123. Gjurić M, Wigand ME, Wolf SR. Enlarged middle fossa vestibular schwannoma surgery: experience with 735 cases. Otol Neurotol 2001;22(2):223-230.
124. Kumon Y, Sakaki S, Kohno K, et al. Selection of surgical approaches for small acoustic neurinomas. Surg Neurol 2000;53(1):52-59.
125. Lustig LR, Rifkin S, Jackler RK, Pitts LH. Acoustic neuromas presenting with normal or symmetrical hearing: factors associated with diagnosis and outcome. Am J Otol 1998;19(2):212-218.
126. Kanzaki JO, K, Inoue, Y, Shiobara, R. Hearing preservation surgery in acoustic neuroma patients with normal hearing. Skull Base Surg 1997;7(3):109-113.
127. Gormley WB, Sekhar LN, Wright DC, Kamerer D, Schessel D. Acoustic neuromas: results of current surgical management. Neurosurgery 1997;41(1):50-58.
128. Weber PC, Gantz BJ. Results and complications from acoustic neuroma excision via middle cranial fossa approach. Am J Otol 1996;17(4):669-675.
129. Post KD, Eisenberg MB, Catalano PJ. Hearing preservation in vestibular schwannoma surgery: what factors influence outcome? J Neurosurg 1995;83(2):191-196.
130. Pollock BE, Lunsford LD, Kondziolka D, et al. Outcome analysis of acoustic neuroma management: a comparison of microsurgery and stereotaxic radiosurgery. Neurosurgery 1995;36(1):215-224.
131. Dornhoffer JL, Helms J, Hoehmann DH. Hearing preservation in acoustic tumor surgery: results and prognostic factors. Laryngoscope 1995;105(2):184-187.
132. Kanzaki J, O-Uichi T, Ogawa K, Shiobara R, Toya S. Hearing preservation by the extended and nonextended middle cranial fossa approach for acoustic neuroma. Skull Base Surg 1994;4(2):76-81.
133. Brookes GB, Woo J. Hearing preservation in acoustic neuroma surgery. Clin Otolaryngol Allied Sci 1994;19(3):204-214.
134. Glasscock 3rd ME, Hays JW, Minor LB, Haynes DS, Carrasco VN. Preservation of hearing in surgery for acoustic neuromas. J Neurosurg 1993;78(6):864-870.
135. Goel A, Sekhar LN, Langheinrich W, Kamerer D, Hirsch B. Late course of preserved hearing and tinnitus after acoustic neurilemoma surgery. J Neurosurg 1992;77(5):685-689.
136. Fischer G, Fischer C, Remond J. Hearing preservation in acoustic neurinoma surgery. J Neurosurg 1992;76(6):910-917.
137. Sughrue ME, Kaur R, Rutkowski MJ, et al. A Critical evaluation of vestibular schwannoma surgery for patients younger than 40 years of age. Neurosurgery 2010;67(6):1646-1653.
138. Quist TS, Givens DJ, Gurgel R, Chamoun R, Shelton C. Hearing preservation after middle fossa vestibular schwannoma removal: are the results durable? Otolaryngol Head Neck Surg 2015;152(4):706-711.
139. Stangerup SE, Caye-Thomasen P, Tos M, Thomsen J. Change in hearing during ‘wait and scan’ management of patients with vestibular schwannoma. J Laryngol Otol 2008;122(7):673-681.
140. Sughrue ME, Yang I, Aranda D, Kane AJ, Parsa AT. Hearing preservation rates after microsurgical resection of vestibular schwannoma. J Clin Neurosci 2010;17(9):1126-1129.
141. Ansari SF, Terry C, Cohen-Gadol AA. Surgery for vestibular schwannomas: a systematic review of complications by approach. Neurosurg Focus 2012;33(3):E14.
142. Charabi S. Acoustic neuroma (vestibular schwannoma): growth and surgical and nonsurgical consequences of the wait-and-see policy. Otolaryngol Head Neck Surg 1995;113(1):5-14.
143. Massick DD, Welling DB, Dodson EE, et al. Tumor growth and audiometric change in vestibular schwannomas managed conservatively. Laryngoscope 2000;110(11):1843-1849.
144. Walsh RM, Bath AP, Bance ML, Keller A, Rutka JA. Consequences to hearing during the conservative management of vestibular schwannomas. Laryngoscope 2000;110(2):250-255.
145. Bozorg Grayeli A, Kalamarides M, Ferrary E, et al. Conservative management versus surgery for small vestibular schwannomas. Acta Otolaryngol 2005;125(10):1063-1068.
146. Caye-Thomasen P, Dethloff T, Hansen S, Stangerup SE, Thomsen J. Hearing in patients with intracanalicular vestibular schwannomas. Audiol Neurootol 2007;12(1):1-12.
147. Quaranta N, Baguley DM, Moffat DA. Change in hearing and tinnitus in conservatively managed vestibular schwannomas. Skull Base 2007;17(4):223-228.
148. Ferri GG, Modugno GC, Pirodda A, Fioravanti A, Calbucci F, Ceroni AR. Conservative management of vestibular schwannomas: an effective strategy. Laryngoscope 2008;118(6):951-957.
149. Pennings RJ, Morris DP, Clarke L, Allen S, Walling S, Bance ML. Natural history of hearing deterioration in intracanalicular vestibular schwannoma. Neurosurgery 2011;68(1):68-77.
150. Fayad JN, Semaan MT, Lin J, Berliner KI, Brackmann DE. Conservative management of vestibular schwannoma: expectations based on the length of the observation period. Otol Neurotol 2014;35(7):1258-1265.
151. Sughrue ME, Kane AJ, Kaur R, et al. A prospective study of hearing preservation in untreated vestibular schwannomas. J Neurosurg 2011;114(2):381-385.
152. Carlson ML, Tveiten OV, Driscoll CL, et al. Long-term quality of life in patients with vestibular schwannoma: an international multicenter cross-sectional study comparing microsurgery, stereotactic radiosurgery, observation, and nontumor controls. J Neurosurg 2015;122(4):833-842.
153. Smouha EE, Yoo M, Mohr K, Davis RP. Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. Laryngoscope 2005;115(3):450-454.
154. Sughrue ME, Yang I, Aranda D, et al. The natural history of untreated sporadic vestibular schwannomas: a comprehensive review of hearing outcomes. J Neurosurg 2010;112(1):163-167.
155. Maniakas A, Saliba I. Microsurgery versus stereotactic radiation for small vestibular schwannomas: a meta-analysis of patients with more than 5 years’ follow-up. Otol Neurotol 2012;33(9):1611-1620.
156. Maniakas A, Saliba I. Conservative management versus stereotactic radiation for vestibular schwannomas: a meta-analysis of patients with more than 5 years’ follow-up. Otol Neurotol 2012;33(2):230-238.
157. Yamakami I, Uchino Y, Kobayashi E, Yamaura A. Conservative management, gamma-knife radiosurgery, and microsurgery for acoustic neurinomas: a systematic review of outcome and risk of three therapeutic options. Neurol Res 2003;25(7):682-690.
158. Shin YJ, Lapeyre-Mestre M, Gafsi I, et al. Neurotological complications after radiosurgery versus conservative management in acoustic neuromas: a systematic review-based study. Acta Otolaryngol 2003;123(1):59-64.
159. Kaylie DM, Horgan MJ, Delashaw JB, McMenomey S. A meta-analysis comparing outcomes of microsurgery and gamma knife radiosurgery. Laryngoscope 2000;110(11):1850-1856.
160. Mangham CA. Retrosigmoid versus middle fossa surgery for small vestibular schwannomas. Laryngoscope 2004;114(8):1455-1461.
161. Carlson ML, Tveiten OV, Driscoll CL, et al. What drives quality of life in patients with sporadic vestibular schwannoma? Laryngoscope 2014.
162. Tveiten OV, Carlson ML, Goplen F, Vassbotn F, Link MJ, Lund-Johansen M. Long-term auditory symptoms in patients with sporadic vestibular schwannoma: an international cross-sectional study. Neurosurgery 2015;77(2):218-227.
163. Harner SG, Fabry DA, Beatty C. Audiometric findings in patients with acoustic neuroma. Am J Otol 2000;21(3):405-411.
164. Carlson ML, Breen JT, Driscoll C, et al. Cochlear implantation in patients with neurofibromatosis type 2: variables affecting auditory performance. Otol Neurotol 2012;33(5):853-862.
165. Lassaletta L, Aristegui M, Medina M, et al. Ipsilateral cochlear implantation in patients with sporadic vestibular schwannoma in the only or best hearing ear and in patients with NF2. Eur Arch Otorhinolaryngol 2016;273(1)27-35.
166. Lloyd SK, Glynn FJ, Rutherford SA, et al. Ipsilateral cochlear implantation after cochlear nerve preserving vestibular schwannoma surgery in patients with neurofibromatosis type 2. Otol Neurotol 2014;35(1):43-51.
167. Hassepass F, Arndt S, Aschendorff A, Laszig R, Wesarg T. Cochlear implantation for hearing rehabilitation in single-sided deafness after translabyrinthine vestibular schwannoma surgery. Eur Arch Otorhinolaryngol 2016;273(9):2373-2383.
168. Kemink JL, Langman AW, Niparko JK, Graham MD. Operative management of acoustic neuromas - the priority of neurologic function over complete resection. Otolaryngol Head Neck Surg 1991;104(1):96-99.
169. Monfared A, Corrales E, Theodosopoulos P, et al. Facial nerve outcome and tumor control rate as a function of degree of resection in treatment of large acoustic neuromas: preliminary report of the acoustic neuroma subtotal resection study. Neurosurgery 2016;79(2)194-203.
170. Raftopoulos C, Abu Serieh B, Duprez T, Docquier MA, Guérit JM. Microsurgical results with large vestibular schwannomas with preservation of facial and cochlear nerve function as the primary aim. Acta Neurochir (Wien) 2005;147(7):697-706.
171. Flickinger JC, Kondziolka D, Niranjan A, Lunsford LD. Results of acoustic neuroma radiosurgery: an analysis of 5 years’ experience using current methods. J Neurosurg 2013;119:3-8.
172. Scheller C, Wienke A, Tatagiba M, et al. Prophylactic nimodipine treatment for cochlear and facial nerve preservation after vestibular schwannoma surgery: a randomized multicenter phase III trial. J Neurosurg 2016;124(3):657-664.
173. Scheller C, Wienke A, Wurm F, et al. Neuroprotective efficacy of prophylactic enteral and parenteral nimodipine treatment in vestibular schwannoma surgery: a comparative study. J Neurol Surg A Cent Eur Neurosurg 2014;75(4):251-258.
174. Brackmann DE, House 3rd JR, Hitselberger WE. Technical modifications to the middle fossa craniotomy approach in removal of acoustic neuromas. Am J Otol 1994;15(5):614-619.
175. Aronzon AR, Ruckenstein MJ, Bigelow DC. The efficacy of corticosteroids in restoring hearing in patients undergoing conservative management of acoustic neuromas. Otol Neurotol 2003;24(3):465-468.
176. Kandathil CK, Dilwali S, Wu CC, et al. Aspirin intake correlates with halted growth of sporadic vestibular schwannoma in vivo. Otol Neurotol 2014;35(2):353-357.
177. Plotkin SR, Merker VL, Halpin C, et al. Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: a retrospective review of 31 patients. Otol Neurotol 2012;33(6):1046-1052.
178. Gao X, Zhao Y, Stemmer-Rachamimov AO, et al. Anti-VEGF treatment improves neurological function and augments radiation response in NF2 schwannoma model. Proc Natl Acad Sci U S A 2015;112(47):
© Congress of Neurological Surgeons
Source: Neurosurgery, February 2018