Guidelines on the Management of Patients with Vestibular Schwannoma
2. Otologic and Audiologic Screening for Patients with Vestibular Schwannomas
download pdf Neurosurgery, 2017
Sponsored by: Congress of Neurological Surgeons (CNS) and the AANS/CNS Tumor Section
Endorsed by: Joint Guidelines Committee of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS)
Authors:
Alex D. Sweeney, MD1,2, Matthew L. Carlson, MD3,4, Neil T. Shepard, PhD3, D. Jay McCracken, MD5, Esther X. Vivas, MD6, Brian A. Neff, MD3,4, Jeffrey J. Olson, MD5
1Bobby R. Alford Department of Otolaryngology-Head and Neck Surgery, Baylor College of Medicine, Houston, Texas, USA
2Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, USA
3Department of Otorhinolaryngology, Mayo Clinic School of Medicine, Rochester, Minnesota, USA
4Department of Neurosurgery, Mayo Clinic School of Medicine, Rochester, Minnesota, USA
5Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia, USA
6Department of Otolaryngology-Head and Neck Surgery, Emory University School of Medicine, Atlanta, Georgia, USA
Correspondence:
Alex D. Sweeney, M.D.
Assistant Professor and Dorothy L. McGee Endowed Chair
Bobby R. Alford Department of Otolaryngology – Head and Neck Surgery
Assistant Professor, Department of Neurosurgery
Baylor College of Medicine
1 Baylor Plaza
Mail Stop – NA102
Houston, TX, 77030
Telephone: 713-798-5900, Fax: 713-798-5841
E-mail: alex.sweeney@bcm.edu
Keywords: Acoustic neuroma, audiologic screening, otologic screening, vestibular schwannoma, skull base surgery
No part of this manuscript has been published or submitted for publication elsewhere.
Abbreviations
ABR: Auditory brainstem response
ASNHL: Asymmetric sensorineural hearing loss
CPA: Cerebellopontine angle
CT: Computed tomography
IAC: Internal auditory canal
MRI: Magnetic resonance imaging
NPV: Negative predictive value
PPV: Positive predictive value
SSNHL: Sudden sensorineural hearing loss
VS: Vestibular schwannoma
Abstract
Question 1
What is the expected diagnostic yield for vestibular schwannomas when using an MRI to evaluate patients with previously published definitions of asymmetric sensorineural hearing loss?
Target population
These recommendations apply to adults with asymmetric sensorineural hearing loss on audiometric testing.
Recommendation
Level 3: On the basis of an audiogram, it is recommended that MRI screening on patients with > 10 dB of interaural difference at 2 or more contiguous frequencies or ≥ 15 dB at one frequency be pursued to minimize the incidence of undiagnosed vestibular schwannomas. However, selectively screening patients with ≥ 15 dB of interaural difference at 3000 Hz alone may minimize the incidence of MRIs performed that do not diagnose a vestibular schwannoma.
Question 2
What is the expected diagnostic yield for vestibular schwannomas when using an MRI to evaluate patients with asymmetric tinnitus, as defined as either purely unilateral tinnitus or bilateral tinnitus with subjective asymmetry?
Target population
These recommendations apply to adults with subjective complaints of asymmetric tinnitus.
Recommendation
Level 3: It is recommended that MRI be used to evaluate patients with asymmetric tinnitus. However, this practice is low yield in terms of vestibular schwannoma diagnosis (< 1%).
Question 3
What is the expected diagnostic yield for vestibular schwannomas when using an MRI to evaluate patients with a sudden sensorineural hearing loss?
Target population
These recommendations apply to adults with a verified sudden sensorineural hearing loss on an audiogram.
Recommendation
Level 3: It is recommended that MRI be used to evaluate patients with a sudden sensorineural hearing. However, this practice is low yield in terms of vestibular schwannoma diagnosis (< 3%).
Introduction
Rationale
Despite considerable evolution in the methods of VS management over the past century, the optimal screening strategy for patients suspected of having a tumor remains unclear. The sensitivity of contrast-enhanced high-resolution MRI to detect retrocochlear pathology and the wide availability of this modality in the present day have led to it becoming the standard for VS identification.1 However, knowing when MRI is indicated can be challenging in the absence of clear neurologic deficits. Additionally, rising health care costs have inspired analysis of resource utilization in a variety of different settings where screening tests are traditionally employed.2–4 Undoubtedly, indiscriminate screening for VSs would have unfavorable financial ramifications given the rarity of these tumors; however, a widely accepted, symptom-based screen to identify patients “at risk” for VS diagnosis continues to be elusive.
Because of the proximity of VS tumors to the essential neural elements of auditory, vestibular, and facial nerve function, initial efforts to create an effective screening protocol have been facilitated by a seemingly predictable symptom profile. Regardless of exact site of origin for most VS tumors,5,6 progressive tumor growth in the internal auditory canal (IAC) and cerebellopontine angle (CPA) would be expected to cause dysfunction in the surrounding structures. Specifically, function of the vestibular and cochlear nerves would be expected to decline in an objective fashion, leading to measurable sensorineural hearing loss and vestibulopathy. Therefore, most tumor screening algorithms have focused on vestibulocochlear function, knowing that a sporadic, unilateral VS should be suspected in the setting of asymmetric dysfunction. However, it has become clear that functional loss associated with VS growth is not always predictable.7
Objectives
This task force aimed to analyze the predictive value of different audiologic symptoms and findings as they relate to VS diagnosis. Significant variability exists in the literature with regard to screening protocols, particularly with respect to the degree of hearing loss necessary to consider pure tone thresholds sufficiently asymmetric.8 Optimally, a set of otologic and audiologic characteristics should be clarified to help identify patients with VSs based on presenting symptoms. The ideal protocol would minimize the probability of either a missed tumor diagnosis (false negative screen) or an unremarkable scan (false positive screen). To achieve these objectives, the following questions were addressed:
- What is the expected diagnostic yield for VSs when using MRI to evaluate patients with previously published definitions of ASNHL?
- What is the expected diagnostic yield for VSs when using MRI to evaluate patients with asymmetric tinnitus, as defined as either purely unilateral tinnitus or bilateral tinnitus with subjective asymmetry?
- What is the expected diagnostic yield for VSs when using MRI to evaluate patients with a SSNHL?
Methods
Writing Group and Question Establishment
The Joint Tumor Section of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS) identified VS management as a topic worthy of guideline development. Members of the Tumor Section and other neurosurgeons and members of other specialties commonly involved in the management of VSs were identified to form the Vestibular Schwannoma Evidence-Based Practice Guideline Task Force (ie, the “task force”). The writers were then divided up into topic sections and developed pertinent questions for those topics. These were circulated among the entire task force, modified, and agreed upon. With these questions in hand, the literature searches, such as the one described below, were executed. Additional details regarding the literature search and review methodology can be found in the Introduction and Methodology Chapter (here). This guideline was then developed using multiple iterations of written review conducted by the authors, then by members of the task force, and finally by AANS/CNS Joint Guideline Committee (JGC).
Search Strategies
The authors collaborated with a medical librarian to search for articles published between January 1, 1990 and December 31, 2014. Three electronic databases were searched (PubMed, EMBASE, and Web of Science). Strategies for searching electronic databases were constructed by the evidence-based clinical practice guideline taskforce members and the medical librarian using previously published search strategies to identify relevant studies (Figure 1 and Table 1).
Study Selection and Eligibility Criteria
Eight hundred and six citations were manually reviewed by the task force with specific inclusion and exclusion criteria as outlined below. Two independent reviewers reviewed and abstracted full-text data for each article, and the 2 sets of data from each reviewer were compared for agreement by a third party. Inconsistencies were re-reviewed and disagreements were resolved by consensus. Citations that considered the audiologic symptom profile of patients with VSs were considered. To be included in this guideline, an article had to be a report with key study parameters including:
- Investigated patients suspected of having VS
- Human subjects
- Was not an in vitro study
- Was not a biomechanical study
- Was not performed on cadavers
- Published between January 1, 1990 and December 31, 2014
- Published in a peer-reviewed journal
- Was not a meeting abstract, editorial, letter, or commentary
- Was published in English
- Quantitatively presented results
Additional inclusion criteria:
- Investigated patients diagnosed with a VS either radiographically (ie, a contrast-enhanced MRI or a heavily weighted T2 sequence (ie, FIESTA sequences) was used for diagnosis of the tumor) or histopathologically (ie, VS was identified on surgical pathology, regardless of the imaging findings)
- Involved a distinct analysis of VS patients in reviews that included various pathologies of the IAC and CPA
- Verified pure tone thresholds and word recognition with formal audiometry
- Included at least 30 patients
The authors supplemented searches of electronic databases with manual screening of the bibliographies of all retrieved publications. The authors also searched the bibliographies of recent systematic reviews and other review articles for potentially relevant citations. All articles identified were subject to the study selection criteria listed above. As noted above, the guideline committee also examined lists of included and excluded studies for errors and omissions. The authors went to great lengths to obtain a complete set of relevant articles to ensure that the guideline is not based on a biased subset of articles. The authors did not include systematic reviews, guidelines, or meta-analyses conducted by others. These documents were developed using different inclusion criteria than those specified in our guideline. Therefore, they may have included studies that do not meet our inclusion criteria. The authors recalled these documents if their abstract suggested that they might address one of our recommendations, and searched their bibliographies for additional studies.
Data Collection Process
Evidence tables for the 3 questions outlined above were constructed using key study parameters as previously described. During the development process, the panel participated in a series of conference calls and meetings.
Classification System and Recommendation Formulation
The concept of linking evidence to recommendations has been further formalized by the American Medical Association (AMA) and many specialty societies, including the American Association of Neurological Surgeons (AANS), the Congress of Neurological Surgeons (CNS), and the American Academy of Neurology (AAN). This formalization involves the designation of specific relationships between the strength of evidence and the strength of recommendations to avoid ambiguity. In the paradigm for diagnostic work, evidence is classified into that which is derived from ≥1 well-designed clinical studies of a diverse population using a “gold standard” reference test in a blinded evaluation appropriate for the diagnostic applications and enabling the assessment of sensitivity, specificity, positive predictive values (PPVs) and negative predictive values (NPVs), and, where applicable, likelihood ratios or class I evidence. Class I evidence is used to support recommendations of the strongest type, defined as level 1 recommendations, indicating a high degree of clinical certainty. Class II evidence is that which is provided by ≥1 well-designed clinical studies of a restricted population using a “gold standard” reference test in a blinded evaluation appropriate for the diagnostic applications and enabling the assessment of sensitivity, specificity, PPVs and NPVs, and, where applicable, likelihood ratios. Class II evidence is used to support recommendations defined as level 2, reflecting a moderate degree of clinical certainty. Class III evidence is that which is provided by expert opinion or studies that do not meet the criteria for the delineation of sensitivity, specificity, PPVs and NPVs, and, where applicable, likelihood ratios. Class III is used to support level 3 recommendations, reflecting unclear clinical certainty. A basis for these guidelines can be viewed online (Guideline Development Methodology)
Results
Question 1
What is the expected diagnostic yield for VSs when using MRI to evaluate patients with previously published definitions of ASNHL?
Target population
These recommendations apply to adults with ASNHL on audiometric testing.
Recommendation
Level 3: On the basis of an audiogram, it is recommended that MRI screening on patients with > 10 dB of interaural difference at 2 or more contiguous frequencies or > 15 dB at one frequency be pursued to minimize the incidence of undiagnosed vestibular schwannomas. However, selectively screening patients with > 15 dB of interaural difference at 3000 Hz alone may minimize the incidence of MRIs performed that do not diagnose a vestibular schwannoma.
Study Selection
A total of 806 studies were screened and assessed for eligibility per the previous criteria, and 17 publications were included in the final review.7,9–24 Pure tone audiometry was the basis of the recommendations in this section, and audiometric definitions of interaural asymmetry were evaluated. In order to be included as a part of this recommendation, a study had to provide a cohort of patients who were screened with MRI having met specific audiometric criteria. In addition, the criteria for screening had to be clearly described for pure tone thresholds, and an analysis had to have been performed regarding the sensitivity and specificity of the criteria. In cases where an authoring center published multiple papers that met these criteria, only the study with the largest number of subject patients was used to avoid duplicate reporting of patient data, if the patient recruitment dates overlapped. Using all of these criteria, a final total of 2 studies were included for analysis.10,11 Data extraction included study design, level of evidence, number of patients, criteria for audiologic screening, and results of the screening method.
Results of Individual Studies
There were 2 studies that met inclusion criteria for this recommendation.10,11 Both studies represent class III data, primarily because of the lack of a blinded assessment and the absence of a validation set. In general, both studies compared audiometric data from their respective cohorts to previously published audiometric screening criteria, as listed below:
- Interaural asymmetry of ≥20 dB at 2 contiguous frequencies.
- Average (1–8 kHz) interaural asymmetry of ≥15 dB.
- Average (1–8 kHz) interaural asymmetry of ≥5 dB.
- Interaural asymmetry ≥15 dB at 2 contiguous frequencies (0.25–8 kHz) if the pure tone average (0.5–4 kHz) in the better ear is <30 dB. If the pure tone average in the better ear is ≥30 dB, asymmetry ≥20 dB at 2 contiguous frequencies is used.
- Males: average (1–8 kHz) interaural asymmetry of ≥20 dB; females: asymmetry at 4 kHz ≥20 dB
- Average interaural asymmetry ≥15 dB (0.25–8 kHz)
- Interaural asymmetry ≥15 dB at any frequency (0.5–4 kHz), or interaural asymmetry of word recognition score of ≥20%, or unilateral tinnitus.
- Interaural asymmetry ≥15 dB at 3 kHz (3000 Hz)
- Interaural asymmetry of ≥20 dB at any single frequency between 0.4 and 4 kHz
- Average (0.5–3 kHz) interaural asymmetry ≥15 dB
- Average (0.5–8 kHz) interaural asymmetry of ≥15 dB
- Interaural asymmetry of ≥10 dB at 2 or more contiguous frequencies, or ≥15 dB at any single frequency
- Interaural asymmetry of ≥15 dB at 2 or more contiguous frequencies, or ≥15% difference in discrimination
The key results of individual studies are outlined in the evidence table (Table 2) and are summarized within the guideline recommendations. Moreover, supplemental statistical data can be found in Table 3.
In 2010, Gimsing11 performed a retrospective review of VS patients that presented to a single center in Denmark between 1973 and 2008. The intent of this work was to provide an audiometric analysis of tumor patients and nontumor patients with objective, asymmetric hearing. Two groups were formed after contrast-enhanced MRI screening: patients that were ultimately diagnosed with a VS, and patients without a VS but with a symptom profile suspicious for a tumor. Two hundred and three tumor patients were identified while 225 patients were in the nontumor comparison group. Only 199 of 203 tumor patients had an audiogram available for review. Of note, it was reported that 24 of the tumor patients were diagnosed as an “incidental finding”—in other words, the tumor was not suspected on the basis of the symptom profile. The findings of this study suggested that the highest sensitivity for tumor diagnosis among all patients with a VS was 93%, which was achieved with either of the following 2 criteria:
- Interaural asymmetry >15 dB at any frequency (0.5–4 kHz), or interaural asymmetry of word recognition score of ≥20%, or unilateral tinnitus.
- Interaural asymmetry of ≥20 dB asymmetry at 2 contiguous frequencies.
On the basis of the criteria analyzed in this study, the highest specificity for tumor diagnosis among all patients with a VS was 52%, which was found with the following criteria:
- Males: average interaural asymmetry >19 dB (1–8 kHz); females: interaural asymmetry at 4 kHz >19 dB
In conclusion, the author reports that the best screening criteria, representing the best combination of sensitivity and specificity, would be either:
- Interaural asymmetry ≥20 dB asymmetry at 2 contiguous frequencies or unilateral tinnitus
- Interaural asymmetry ≥15 dB at any 2 frequencies between 2 and 8 kHz
In both 2009 and 2011, Saliba et al9,10 described a single center’s experience with audiometric criteria for VS screening between the years of 2003 to 2007 and 2003 to 2008, respectively. As per the aforementioned criteria, the entire first study (2009) was excluded from this recommendation to avoid duplicate reporting of the same patient population. In the 2011 work, the authors performed a retrospective chart review of patients who underwent a screening MRI when a symptom profile was suggestive of a VS. In total, 212 patients were analyzed, 84 of whom were found to have a tumor. Based on the following criteria analyzed in this study, the highest sensitivity and NPV for tumor diagnosis amongst all patients with a VS was approximately 93% and 80%, respectively:
- Interaural asymmetry ≥10 dB at 2 or more contiguous frequencies or ≥15 dB at any single frequency
The highest sensitivity for tumor diagnosis amongst all patients with a VS was 76% in this study, which was found with the following criterion:
- Interaural asymmetry ≥15 dB at 3000 Hz
The highest PPV for tumor diagnosis among all patients with a VS was 86% in this study, which was found with the following criterion:
- Interaural asymmetry ≥15 dB at 3000 Hz
The highest positive likelihood ratio for tumor diagnosis among all patients with a VS was 2.91 in this study, which was found with the following criterion:
- Interaural asymmetry ≥15 dB at 3000 Hz.
In conclusion, the authors report that the “rule of 3000” (interaural asymmetry ≥15 dB at 3000 Hz) offers the most cost-effective audiometric screening criterion for VS diagnosis.
These 2 studies examine the utility of different audiologic screening methods for VSs by analyzing cohorts of patients with proven interaural asymmetry. Tumor diagnosis was made with contrast-enhanced MRI. The most sensitive criteria are those with the most permissive definitions of asymmetry, notably interaural asymmetry ≥10 dB at 2 or more contiguous frequencies or ≥15 dB at any single frequency. However, the most specific screening method with the highest PPV was an interaural asymmetry of ≥15 dB at 3 000 Hz.
Risks and Bias of Study Limitations
When analyzing retrospective reviews of screening paradigms from different tertiary care centers, selection bias has to be considered. In general, it is possible that some VS patients were not effectively captured with screening, and not all patients who met criteria were able or willing to complete an MRI. Therefore, data analysis may not truly reflect all VS cases. In addition, spectrum bias has to be considered for any study conducted through a tertiary referral otology/audiology specialty. If an authoring center offers expertise in the management of hearing loss, it is likely that their patient population is not necessarily representative of the general population. Specifically, there may be a disproportionate number of patients with hearing complaints relative to the general population. Moreover, patients with asymmetric hearing loss in a given population may not always be referred from a primary care clinic to a tertiary care clinic, or it may be possible that certain patients in a given population are referred to another tertiary care center.
Synthesis of Results
Evidence suggests that for the diagnosis of a VS, the most sensitive, current audiometric definition of ASNHL is ≥10 dB at 2 or more contiguous frequencies or ≥15 dB at any single frequency. However, the criterion with the highest PPV defines asymmetry as ≥15 dB interaural asymmetry at 3000 Hz.
Discussion
The ideal audiometric screening protocol for VSs continues to be an area of interest, particularly in an era when high-resolution MRI is increasingly available, and resource utilization is becoming increasingly scrutinized. ASNHL is generally believed to be the most common symptom reported by patients with a VS.9–11 Because MRI has become the gold standard method of diagnosis,1 logic would dictate that any patient with an ASNHL would be screened with an MRI when identified. However, a cost-conscious medical practice would encourage a compromise between the most sensitive and specific screening criteria. On one hand, the most effective method of screening would involve the broadest definition of asymmetric hearing loss in order to mitigate the risk of a “missed” tumor. To this end, screening any patient with an interaural asymmetry ≥10 dB at 2 or more contiguous frequencies or ≥15 dB at any single frequency would allow a physician to have a evidence-based algorithm that is the least likely to result in undiagnosed tumors. On the other hand, the most efficient screening method would result in the smallest number of negative scans. Using the “rule of 3000” as an audiometric screening protocol would be an evidence-based strategy that would ensure the highest predictive value for MRI. To reconcile these differences, physicians searching for an appropriate screening protocol for their respective practices would first need to clearly define their screening philosophy in light of available resources: is it more important to have fewer false negative screens or fewer false positive screens?
Although most tumor patients present with an ASNHL, it cannot be ignored that most cases of ASNHL are unlikely to be ultimately attributed to a VS. Focusing on the incidence of false-positive screens in a given population with sensorineural hearing loss, the difficulty inherent in the establishment of a standardized audiometric screening protocol for VSs becomes readily apparent. Primarily, although VSs are generally felt to represent the most common neoplasm of the CPA, there are other identifiable pathologies that could conceivably cause an ASNHL. For example, CPA meningiomas, vascular anatomic variants, and ischemic events have all been identified as potential sensorineural hearing loss etiologies.8 Moreover, even when considering all radiographically apparent causes of hearing loss, many reports suggest that the diagnostic evaluation for most cases of ASNHL will not reveal a causative pathology.25–31 In this scenario, it might be reasonable to consider that the most reliable screening algorithm for VSs may incorporate other audiometric findings or even other elements of a patient’s subjective and objective evaluation. The significance of SSNHL and asymmetric tinnitus, in particular, will be addressed elsewhere in this paper. A history of noise-induced hearing loss (NIHL) deserves special mention, particularly considering the possibility that the number of patients with this particular complaint may be increasing over time.32 The propensity toward asymmetry in NIHL has been previously described, although the pathophysiologic basis of this finding is unclear.33,34 When considering the high rate of false-positive screens in the 2 studies reviewed for this portion of the recommendation, one question would be whether or not a reported history of noise exposure would be a negative predictive factor for ultimate tumor diagnosis. Other findings that may bear relevance are the report of subjective dizziness and the presence of asymmetric low frequency hearing loss. In 2009, Saliba et al9 reported that complaints of subjective dizziness were a negative predictive factor (P = .001) for an ultimate tumor diagnosis, while in 2010, Gimsing11 reported that “reverse slope” pure tone audiogram trajectories, in which low frequency hearing loss was predominant, were also a negative predictive factor for tumor diagnosis (P < .01). As work proceeds toward the development of a more specific audiometric screening protocol for VS diagnosis, factors beyond simple pure tone asymmetry will likely need to be considered.
Question 2
What is the expected diagnostic yield for VSs when using MRI to evaluate patients with asymmetric tinnitus, as defined as either purely unilateral tinnitus or bilateral tinnitus with subjective asymmetry?
Target population
These recommendations apply to adults with subjective complaints of asymmetric tinnitus.
Recommendation
Level 3: It is recommended that MRI be used to evaluate patients with asymmetric tinnitus. However, this practice is low yield in terms of vestibular schwannoma diagnosis (<1%).
Study Selection
A total of 806 studies were screened and assessed for eligibility per the previous criteria, and 17 publications were included in the final review.7,9–24 This recommendation evaluated the utility of asymmetric tinnitus as a screening tool by analyzing both the association of asymmetric tinnitus in the general population with the diagnosis of a VS and the frequency with which tumor patients retrospectively reported asymmetric tinnitus at the time of their presentation. Therefore, the presence of subjective, asymmetric tinnitus was specifically considered in this recommendation, and studies were included only if they analyzed asymmetric tinnitus as a solitary symptom or as part of a symptom profile in a patient screened for or diagnosed with a VS. With the application of this exclusion criteria, 8 studies were included.7,9,11,14–16,22,24 Data extraction included study design, level of evidence, number of patients, number of tumors found in the setting of asymmetric tinnitus, and if applicable, the number of tumor patients with complaints of asymmetric tinnitus. In cases where an authoring center published multiple papers on this subject, only the study with the largest number of subject patients was used to avoid duplicate reporting of patient data.
Results of Individual Studies
Of the 8 studies analyzed, 2 examined the incidence of asymmetric tinnitus as a solitary audiologic symptom.7,14 All studies were thought to represent class III data primarily because of the lack of a blinded assessment and the absence of a validation set. Specific data from each publication can be found in Tables 2 and 4.
In 1998, Lustig et al7 performed a retrospective review of all patients diagnosed with VS at a single center between 1983 and1996 in order to describe the symptoms of patients who presented without an ASNHL. In total, 29 of 546 tumor patients presented with symmetric sensorineural hearing, defined as the absence of any of the following: interaural asymmetry ≥15 dB at a single frequency or ≥10 dB at 2 or more frequencies (500 Hz and 1, 2, and 4 kHz), speech reception threshold (SRT) ≥20 dB, or an interaural speech discrimination score differential of ≥20%. It is noteworthy that 5 of these 29 symmetric hearing patients had tumors >3 cm in their greatest diameter. The most common symptoms in these 29 patients were disequilibrium (41%) and cranial neuropathies aside from the cochlear nerve, including facial weakness in 34% and facial paresthesia in 10%. Asymmetric tinnitus was reported in 4 patients. Therefore, approximately 0.7% of tumor patients at this single center presented with asymmetric tinnitus in the absence of an objective ASNHL. In a similar fashion, Dawes et al14 described the experience of a single referral center with patients who presented with asymmetric tinnitus. They evaluated 174 patients for this complaint, and all patients were screened with a contrast-enhanced MRI. Out of this group, 1 patient (approximately 0.7%) was found to have a VS.
The remaining 6 studies analyzed the frequency with which patients had a complaint of asymmetric tinnitus at the time of their presentation, regardless of other symptoms.9,11,15,16,22,24 Additional data from these studies can be found in Tables 2 and 5. In 2 of these 6 studies,9,11 rates of asymmetric tinnitus were compared in cohorts diagnosed with VS and in an unmatched comparison group with asymmetric hearing loss in the absence of a tumor. In these studies, no significant difference was found in the incidence of asymmetric tinnitus among tumor patients and nontumor patients.
The remaining studies focused on tumor populations without control/comparison groups. In 2000, Haapaniemi et al22 reported on 41 patients with tumors diagnosed with contrast-enhanced MRI, revealing that 25 of 41 patients (60.9%) reported asymmetric tinnitus at the time of their diagnosis, while 4 patients (9.8%) reported that tinnitus was their initial symptom. In 1996, Neary et al24 retrospectively analyzed the symptom profile of patients who were radiographically or histologically diagnosed with VS. In a cohort of 93 patients, 14 (15.1%) experienced tinnitus at the time of presentation. Also in 1996, Levy et al15 reported on the screening of 118 patients who presented with presumed vestibulocochlear dysfunction, 9 of whom had VS diagnosed by MRI or surgical pathology. Of these 9, there were 6 patients that reported tinnitus as a presenting symptom, and 5 of these patients had an ASNHL documented as well, defined as ≥25 dB or more at 2 or more frequencies between 1 and 8 kHz or ≥20% asymmetry in discrimination. Therefore, 1 patient presented with asymmetric tinnitus in the absence of an asymmetric hearing loss. In 1995, Van Leeuwen et al16 analyzed the 12-year experience at a single center including 164 pathologically proven VSs. Out of this cohort, it was reported that 56.7% presented with asymmetric tinnitus.
Risks of Bias and Study Limitations
As discussed in the prior recommendation, the risks of selection bias and spectrum bias have to be considered when evaluating data presented by a tertiary referral center. However, in addition to what was previously mentioned, this recommendation incorporates data from studies in which tumors were definitively diagnosed with histopathology in addition to those diagnosed with an MRI. Therefore, a new selection bias is assumed in which study data is more likely to be reflective of only patients that were candidates for surgery rather than the VS population as a whole. It therefore stands to reason that the data presented may not be reflective of the entire VS population, considering that some tumors may have been observed or received stereotactic radiosurgery. Publication bias also applies in a similar fashion, given that not all tumor patients seen in a particular center would be included in these studies. Recall bias must also be considered in studies involving a post-treatment analysis that relies on patients to recall if their symptoms were initially present prior to treatment.
Synthesis of Results
These 8 studies examined the association of asymmetric tinnitus with the diagnosis of a VS. Tumor diagnosis was made with contrast-enhanced MRI or with tumor tissue histopathology. In total, there were 720 patients subjected to MRI screening on the basis of asymmetric tinnitus in the absence of asymmetric hearing loss. Five patients from this group were found to have a tumor, suggesting that the prevalence of asymmetric tinnitus as an initial presenting symptom among patients with a VS is <1%. However, many patients with a VS diagnosis report asymmetric tinnitus, irrespective of other symptoms. Of 584 tumors from studies that met inclusion criteria, 319 (54.6%) experienced asymmetric tinnitus. When considering these findings, it would appear that asymmetric tinnitus may correlate more with asymmetric hearing loss, in general, rather than the presence of a tumor. Based on available data, the presence of asymmetric tinnitus is a relatively unreliable screening tool for VSs.
Discussion
The 2014 Clinical Practice Guidelines on tinnitus produced by the AAO-HNS report that as many as 15% of Americans suffer from tinnitus.35 Moreover, it is alleged to be the most common service-related disability in the American veteran population. The symptom of tinnitus occurs when a noise is perceived in the absence of an objectively produced sound, and tinnitus is generally, but not always, associated with an audiometrically measurable sensorineural hearing loss. It can be considered to be “primary” when there is no clear explanation for the tinnitus and “secondary” when there is a recognizable cause.
Despite the relative frequency with which tinnitus is seen in outpatient clinics, the pathophysiology of this complaint remains unclear. Although tinnitus is generally associated with sensorineural hearing loss, not all patients with sensorineural hearing loss experience tinnitus, leading to a variety of mechanisms for production and perception of tinnitus that have been proposed over time.36 Recently, Larson and Cheung37 postulated that the caudate nucleus of the basal ganglia might play an important role in the gating of tinnitus, and that deep brain stimulation in this area my help to modulate this “auditory phantom.”
The general, the association between VSs and tinnitus is fairly well established, with recent Acoustic Neuroma Association (ANA) survey data describing that approximately 60% of tumor patients report tinnitus.38 However, evidence may suggest the causal relationship between the tumor and tinnitus may simply be indirect, or in other words, a byproduct of the sensorineural hearing loss associated with tumors; tumors do not cause tinnitus as much as unilateral, sporadic tumors cause sensorineural hearing loss, which is associated with tinnitus. When assessing tinnitus modulation in relation to patient demographics, tumor characteristics, and management strategy, there are no clear associations that are evident, leading to the recommendation that tumor patients be counseled to effectively disassociate any relationship perceived between their tumor, the treatment of their tumor, and their tinnitus.39
The use of asymmetric tinnitus as a screening tool for VSs is predicated on the assumption that a unilateral, sporadic VS could lead to unilateral hearing loss, and potentially, asymmetric tinnitus. The AAO-HNS recommends further evaluation for tinnitus when it is unilateral or associated with ASNHL, among other circumstances.35 Per the present analysis, it would appear that the use of asymmetric tinnitus as an independent screening tool for VS diagnosis would be expected to produce a marginal increase in the sensitivity of tumor screening protocols. Providers could expect to diagnose a tumor in <1% of cases when asymmetric tinnitus is present in the absence of ASNHL. When considering the broad etiologic possibilities for tinnitus in a given patient, it may be important to formally distinguish between cases of asymmetric tinnitus on the basis of factors that could independently lead to asymmetric tinnitus, such as noise exposure.
Question 3
What is the expected diagnostic yield for VSs when using MRI to evaluate patients with a SSNHL?
Target population
These recommendations apply to adults with a verified SSNHL on an audiogram.
Recommendation
Level 3: It is recommended that MRI be used to evaluate patients with a sudden sensorineural hearing. However, this practice is low yield in terms of vestibular schwannoma diagnosis (<3%).
Study Selection
A total of 806 studies were screened and assessed for eligibility per the previous criteria, and 17 publications were included in the final review.7,9–24 This recommendation evaluated the utility of SSNHL as a screening tool for VS by analyzing both the likelihood of patient presentation with a SSNHL and the frequency with which patients ultimately diagnosed with a tumor reported a SSNHL at the time of their presentation. Therefore, patient presentation with an audiogram-verified SSNHL was specifically considered in this recommendation, and studies were included only if they analyzed sudden hearing loss alone as a screening tool or if they analyzed sudden hearing loss as a presenting symptom in patients that were ultimately diagnosed with a VS. With the application of this exclusion criteria, 10 studies were included.11,12,17–24 Data extraction included study design, level of evidence, number of patients, and the number of tumors found following a sudden ASNHL. In cases where an authoring center published multiple papers on this subject, only the study with the largest number of subject patients was used to avoid duplicate reporting of patient data.
Results of Individual Studies
Of the 10 studies analyzed, there were 2 general classes of articles: those reporting the incidence of tumor diagnosis in a patient cohort experiencing a SSNHL and those reporting the incidence of SSNHL as a presenting symptom in a cohort of known VS patients. The former category included 6 articles, which are outlined in Tables 2 and 6. All studies were thought to represent class III data primarily due to the lack of a blinded assessment and the absence of a validation set.
In 2011, Lee et al20 reported the experience of a single center with SSNHL between 2002 and 2008. Any patient who presented with a 30-dB loss in 3 consecutive frequencies “instantaneously or progressively over several days” was included; however, the authors did not specifically clarify the definition of several days. Of 295 patients with a sudden hearing loss, there were 9 ipsilateral VSs found that were presumed to have caused the hearing loss. In 4 of these 9 cases, there was a reported significant recovery of the sensorineural hearing with an unspecified corticosteroid treatment. In addition to the 9 ipsilateral tumor cases, there were also 3 cases where a VS was identified in the contralateral, better hearing ear. In 2006, Cadoni et al21 described the experience of a single center with 54 cases of SSNHL, defined as a 30-dB threshold shift in 3 contiguous frequencies over 3 days or less. In this cohort, an explanation of the hearing loss was allegedly identified in 6 cases, with 1 of these cases representing an ipsilateral VS. In 2004, Aarnisalo et al19 reviewed the experience from a single center with SSNHL, in which 82 patients were screened with MRI after experiencing an audiometric loss equaling or greater than an average of 25 dB across 3 consecutive frequencies occurring in less than a 3-week period of time. In total, 12 patients were found to have a causal relationship between an MRI finding and the sudden loss, with 4 VSs noted. Other presumably causative etiologies were ischemia, vascular anomalies, and demyelinating disease. Nageris et al,12 in 2003, reviewed a single center’s experience with SSNHL, reporting on cases in which a 10-dB threshold shift was noted in at least 2 frequencies over a undefined, “few” days. Patients were excluded if they were subsequently diagnosed with Meniere disease, a perilymphatic fistula, middle ear disease, external ear disease, or “systemic disease.” With these inclusion and exclusion criteria, 67 patients were analyzed, of whom 24 (36%) had a sudden hearing loss. In 1998, Fitzgerald et al18 studied a single center’s experience with SSNHL, and in this case, they defined it as a 30-dB loss in 3 contiguous frequencies that occurs within a 24- to 72-hour period of time or less. A total of 78 patients were selected for analysis, and 24 of the patients were found to have a probable cause of the hearing loss identified on MRI, and 3 had a VS. Saunders et al,17 in 1995, identified 13 VSs out of 431 SSNHL patients definitively screened with a contrast-enhanced MRI scan. Sudden hearing loss, in this case, was defined as 25 dB at 1 or more frequencies over 48 hours or less.
The remaining studies focused on the retrospective report of SSNHL at the time of diagnosis in established VS patient populations. Of this group, 5 studies were selected for analysis.11,17,22–24 Details from these articles can be found in Tables 2 and 7. In 2010, Gimsing11 found that approximately 10% of VS patients experienced a sudden hearing loss, though the definition of the sudden loss was not clearly defined. In 2005, Sauvaget et al23 reported on 28 of 139 tumor patients who reported a sudden hearing loss prior to their presentation. However, an audiogram verified an actual SSNHL in only 21 cases, and the criteria used to define a sudden loss is not explicitly defined. Haapaniemi et al,22 in 2000, described a cohort of 41 VS patients, of which 5 experienced a sudden hearing loss, and Neary et al,24 in 1996, described 93 VS patients, out of which 7 experienced a sudden hearing loss. In both of these studies, the definition of a sudden hearing loss was not provided. Saunders et al’s 1995 report,17 referenced in the above paragraph, discussing SSNHL as a screening symptom, also included a review of patients who were previously diagnosed with a VS. In this study, 79 of 1204 tumor patients were found to have a documented SSNHL at presentation.
Risks of Bias and Study Limitations
Similar to what has been discussed in the prior recommendations, the risks of selection bias and spectrum bias must be considered when evaluating data presented by a tertiary referral center.
Synthesis of Results
These 10 studies examine the association of SSNHL with the diagnosis of a VS. Tumor diagnosis was established with contrast-enhanced MRI or with tumor tissue histopathology. When used as a screening tool for the general population, 54 tumors were found out of 1007 patients screened, suggesting that the prevalence of SSNHL as a presenting sign for a VS is approximately 5.4%. When considering VS patients who have a documented history of SSNHL, 133 patients of 1680 were identified, suggesting that 7.9% of tumor patients experienced SSNHL before their diagnosis. Based on available studies, SSNHL is a more reliable indicator of the presence of a VS than asymmetric tinnitus in the absence of an associated sensorineural hearing loss.
Discussion
The differential diagnosis for SSNHL is broad. Presumably, any process that interferes with the reception and translation of sound energy in the cochlea through the interpretation of a sound signal in the auditory cortex could result in a sensorineural hearing loss. Therefore, identifying a SSNHL could conceivably implicate vascular, infectious, autoimmune, or neoplastic etiologies, among others. With regard to VSs, the association with SSNHL has long been established, dating back at least to the work of Harvey Cushing.40 However, the spectrum of literature regarding SSNHL in tumor patients suggests that the pathophysiology of this relationship may be multifactorial. For example, it has been postulated that a growing tumor may compress the blood supply of the labyrinthine artery,41 while more recently, a metabolic mechanism for hearing loss has been suggested.42 Moreover, literature reviewed in the present study also indicates that hearing improvement in cases of sudden hearing loss may occur in the setting of a VS.12
Although the studies included in this particular recommendation used different definitions of SSNHL, as well as different methodology, the literature would generally suggest that the yield of contrast-enhanced MRI for VS diagnosis in this setting is fairly low. At face value, the predictive value of SSNHL as a screening tool for VS diagnosis in this recommendation was 8.8% (median 3.6; range 1.9–35.8%). When evaluating Nageris et al12 from 2003, which provided a value significantly higher than the other studies used for this recommendation (35.8%), it is noteworthy that many other etiologies that could potentially account for sudden hearing loss were excluded from this study, including Meniere disease and “systemic disease.” Therefore, the results of that particular study may not be representative of the sudden hearing loss population as a whole. When excluding the findings of this study, the average predictive value drops to 2.8%, suggesting that screening every patient with SSNHL would have a false-positive result approximately 97% of the time. However, given the possibility that an MRI could also identify other sudden hearing loss etiologies other than a VS, contrast-enhanced MRI continues to be a part of the recommended screening algorithm in this clinical setting.43
Summary Discussion
Although a variety of different studies have evaluated the optimal screening methods for VSs, no perfect method exists. In general, most screening paradigms for VSs have a low diagnostic yield. However, the significance of a “positive” finding and the increasing sensitivity and availability of diagnostic tests in the modern era have made it possible and desirable to identify VSs at their smallest and most treatable stage. Yet in some regard, the literature that has led to the creation of tumor screening guidelines has created a conflict of purpose: is the goal of the clinician to “never miss” tumors or to efficiently use limited resources to find tumors? This conflict is distinctly demonstrated when considering the above recommendations. Clearly, the most sensitive screening paradigm based on interaural audiometric threshold asymmetry, asymmetric tinnitus, and ASNHL would incorporate the least stringent of all of these criteria. In other words, MRI screening would be offered to any patient presenting with subjectively asymmetric tinnitus and/or a measurable SSNHL or an interaural asymmetry of ≥10 dB at 2 or more frequencies or ≥15 dB at any single frequency, and it would be expected that this method would have the highest likelihood of diagnosing the greatest number of VSs while also providing the lowest likelihood of missing an opportunity for VS diagnosis. Yet considering only the conflict example presented in the first recommendation, this increase in sensitivity would come at the expense of specificity, leading to a large number of negative MRI scans, and therefore a less efficient utilization of resources. In order to employ the ideal screening criteria for any clinical setting, the goals of the physician must first be clearly delineated.
The process of screening for retrocochlear pathology has evolved over the past century. However, over time, contrast-enhanced MRI has emerged as the gold standard screening method for VS and other IAC/CPA pathology. Prior to the CT and MRI era, patient history and physical examination were the only available tools when a VS was suspected; however, since then, a variety of different radiologic and neurophysiologic tests have emerged to contribute to the diagnostic algorithm for these tumors.1 Although a thorough clinical history and physical examination and audiologic testing remain vital elements in the evaluation of a patient with suspected retrocochlear pathology, the past few decades of VS screening literature have evaluated the sensitivity, specificity, and cost-effectiveness of different screening tests. In particular, auditory brainstem response (ABR), contrast-enhanced MRI, and noncontrast MRI have been a publication focus. The emergence of MRI represents the most recent development in a line of radiologic studies that were designed to target the lateral skull base, starting with plain film radiograph used by Harvey Cushing and progressing through polytomography and CT air cisternography. Similarly, ABR emerged as a primary audiologic assessment tool that was designed to raise suspicion of retrocochlear pathology. In addition, with the increasingly prevalent emphasis placed on cost-conscious and cost-effective diagnostic strategies, ABR became a recommended screening test. However, with the passage of time, the superior sensitivity and specificity of MRI has become clear,1,44 even though the debate continues with regard to the necessity of intravenous contrast.45,46 Ultimately, it seems apparent that the addition of contrast offers a marginal improvement in the sensitivity of MRI screening although it is generally more time-consuming and costly.47,48
When evaluating screening algorithms for VSs, it is important to consider that this tumor type does not encompass all forms of retrocochlear pathology. VSs are the most common benign neoplasm of the CPA. However, in most clinical settings, the performance of an MRI for an ASNHL, SSNHL, or asymmetric tinnitus will identify causative pathology beyond just VSs, which raises the diagnostic yield of the screening test. In 2012, Cheng et al8 performed an analysis of 1751 patients subjected to a screening MRI at a single center. While VSs were the most commonly identified neoplasm (5.09%), nearly 25% of cases involved the identification of a different causative pathology. Moreover, approximately 2% of the cases involved the identification of a CPA/IAC meningioma, or a so-called “acoustic meningioma.” In 2004, Aarnisalo et al19 published a similar report in which MRI was able to establish a causative pathology for sudden hearing loss in 14% of cases, with only 5% attributable to a VS. The remainder of the cases involved vascular pathology and “demyelinating processes.” With these studies as examples, the ability to identify pathology beyond VSs should be a consideration when designing a screening algorithm for otologic symptoms.
Conclusions and Key Issues for Future Investigation
VSs are the most common benign tumors of the CPA. Otologic symptoms, such as hearing loss, are common at presentation for patients ultimately diagnosed with VSs, although as long as these symptoms are the sole criteria for a particular screening guideline, it is reasonable to assume that some tumors will be missed. Moreover, specificity and sensitivity vary among the guidelines, suggesting that the most appropriate recommendation for a given center would depend on the philosophy of the center (ie, is it more important to miss fewer tumors or have fewer negative scans) and available resources.
The existing literature on the expected VS patient symptom profiles suggests that as long as objective audiometric criteria are the basis of any screening protocol for VSs, a portion of tumors will always go undiagnosed. Generally, slow tumor growth rates and the potential for compensation within the IAC and CPA may be a major contributing factor to the failure of a symptom-based screening system. The growth rate of VSs has long since been a matter of interest and speculation, with most reports identifying an average expansion of ≤1 mm per year when growth is observed.49–53 With a slow rate of progression, functional accommodation is possible, limiting the presenting symptom profile even in the setting of very large tumors. Van Leeuwen et al16 demonstrated that it can be difficult to consistently correlate tumor size with associated symptoms. The work of Lustig et al7 reflected on a single center’s experience with VS diagnosis in the setting of relatively symmetric sensorineural hearing, and over half of the 34 reported tumors were found to be >1 cm at diagnosis with 5 tumors being >3 cm. Less typical symptoms that led to a positive diagnosis included disequilibrium/imbalance, other cranial nerve deficits, and headache. In 1998, Moffat et al54 described the Cambridge experience with VS symptom profiles at the time of diagnosis, reporting that 10.7% of patients presented with these “atypical” complaints. Although the scope of this paper was limited to audiometric screening and subjective tinnitus, it stands to reason that the most comprehensive criteria for VS screening would involve multiple features, both in terms of a patient’s symptoms, audiologic testing, and their audiologic history (eg, noise exposure). Research directed toward the development of a weighted “score” for VS diagnosis will be a welcome addition to this body of literature.
Conflict of Interest (COI)
The Vestibular Schwannoma Guidelines Task Force members were required to report all possible COIs prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of Task Force members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methods manuscript.
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons and the Tumor Section of the Congress of Neurological Surgeons and the American Association of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document.
Acknowledgments
The authors acknowledge the Congress of Neurological Surgeons Guidelines Committee for its contributions throughout the development of the guideline and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Guidelines Committee for its review, comments, and suggestions throughout peer review, as well as Trish Rehring, MPH, CHES, and Mary Bodach, MLIS, for their assistance. Throughout the review process, the reviewers and authors were blinded from one another. At this time, the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Sepideh Amin-Hanjani, MD, D. Ryan Ormond, MD, Andrew P. Carlson, MD,
Kimon Bekelis, MD, Stacey Quintero Wolfe, MD, Chad W. Washington, MD,
Cheerag Dipakkumar Upadhyaya, MD, and Mateo Ziu, MD.
Figures
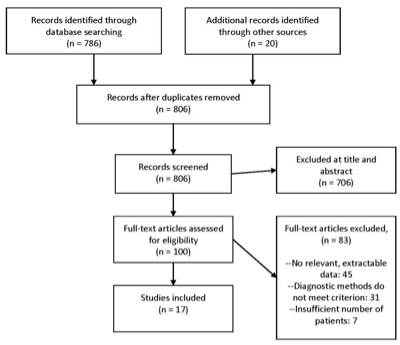
Figure 1. Article flow chart.
Table 1. Audiologic screening primary search strategy, results, and initial pruning
|
ENDNOTE PUBMED (NLM), searched on May 10th, 2015:
|
|
Search 1: All Fields, Contains “acoustic neuroma” OR All fields, Contains “vestibular schwannoma” AND All Fields, Contains “audiometric”
Results: 176
|
|
Search 2: All Fields, Contains “acoustic neuroma” OR All fields, Contains “vestibular schwannoma” AND All Fields, Contains “tinnitus”
Results: 456
|
|
Search 3: All Fields, Contains “acoustic neuroma” OR All fields, Contains “vestibular schwannoma” AND All Fields, Contains “sudden hearing loss”
Results: 183
|
|
Search 4: All Fields, Contains “acoustic neuroma” OR All fields, Contains “vestibular schwannoma” AND All Fields, Contains “asymmetry”
Results: 68
|
|
TOTAL: 883
|
|
ENDNOTE EMBASE, searched on May 10th, 2015:
|
|
Search 1: Abstract, Contains “acoustic neuroma” OR Abstract, Contains “vestibular schwannoma” AND Abstract, Contains “audiometric”
Results: 108
|
|
Search 2: Abstract, Contains “acoustic neuroma” OR Abstract, Contains “vestibular schwannoma” AND Abstract, Contains “tinnitus”
Results: 253
|
|
Search 3: Abstract, Contains “acoustic neuroma” OR Abstract, Contains “vestibular schwannoma” AND Abstract, Contains “sudden hearing loss”
Results: 37
|
|
Search 4: Abstract, Contains “acoustic neuroma” OR Abstract, Contains “vestibular schwannoma” AND Abstract, Contains “asymmetry”
Results: 40
|
|
TOTAL: 438
|
|
ENDNOTE Web of Science, searched on May 10th, 2015:
|
|
Search 1: Title/Keywords/Abstract, Contains “acoustic neuroma” OR Title/Keywords/Abstract, Contains “vestibular schwannoma” AND Title/Keywords/Abstract, Contains “audiometric”
Results: 112
|
|
Search 2: Title/Keywords/Abstract, Contains “acoustic neuroma” OR Title/Keywords/Abstract, Contains “vestibular schwannoma” AND Title/Keywords/Abstract, Contains “tinnitus”
Results: 243
|
|
Search 3: Title/Keywords/Abstract, Contains “acoustic neuroma” OR Title/Keywords/Abstract, Contains “vestibular schwannoma” AND Title/Keywords/Abstract, Contains “sudden hearing loss”
Results: 124
|
|
Search 4: Title/Keywords/Abstract, Contains “acoustic neuroma” OR Title/Keywords/Abstract, Contains “vestibular schwannoma” AND Title/Keywords/Abstract, Contains “asymmetry”
Results: 49
|
|
TOTAL: 528
|
|
Summary of Primary Search
Combined from 3 database searches, total of 1849 candidate articles
Deleted all duplicate articles
Deleted articles published before 1/1/1990 and after 12/31/2014
Total number of candidate articles after primary search = 806
|
Table 2. Evidence table
|
Author/Year
|
Study Description
|
Data Class
|
Results and Conclusion
|
|
Saliba et al, 2011
|
Retrospective review from a single center that analyzed symptoms most predictive of a VS diagnosis on MRI evaluating patients seen between 2003–2008. Patients had to have had an audiogram and a contrast-enhanced MRI. Hearing asymmetry was defined, for purposes of patient screening as 10 dB at ≥1 frequencies or ≥15% discrimination difference. The study was purposefully broad to cover all the definitions of hearing asymmetry in the literature.
|
III
|
Results: Eighty-four of 232 patients meeting the aforementioned criteria had a VS.
For the rule 3000 Hz:
Sensitivity: 0.73
Specificity: 0.76
PPV: 0.86
NPV: 0.68
LR(+): 2.91
LR(−): 0.38
Conclusion: The rule of 3000 (15 dB interaural difference at 3000 Hz) has the highest likelihood ratio for VS diagnosis out of all of the previously reported definitions of asymmetric pure tone thresholds. However, it is important to note that VS detection sensitivity would be diminished with this definition.
|
|
Lee et al, 2011
|
Retrospective review of a single center’s experience with SSNHL from 2002–2008. FIESTA sequence MRI was used to screen any patient with a >30 dB loss in 3 contiguous frequencies over an undefined “several days” or less.
|
III
|
Results: Twelve patients had a VS found out of the 295 screened, but 3 of 12 actually had a tumor incidentally found in the ear contralateral to the hearing loss. For that reason, 9 patients presumably had a SSNHL associated with a VS.
Conclusion: SSNHL is a relatively rare presenting sign of a VS. Approximately 4% of the patients in this current study had a SSNHL. On the basis of these findings, the authors advocate obtaining MRI screening on patients with a SSNHL.
|
|
Gimsing, 2010
|
Retrospective review of a single center’s experience with asymmetric pure tone thresholds and discrimination between 1973–2008. Patients diagnosed with a VS using MRI were included in the study. A control group was also used including patients who had suspicious audiograms or symptoms and no tumor on MRI.
|
III
|
Results: 203 tumor patients were identified (199 had audiograms available, 197 with known tumor size). 225 control patients were identified. These patients were not matched to the tumor patients for any demographic. The mean age was the same in both groups (55 years), and the nontumor group had more men (P < .05)
10% of patients experienced a SSNHL in the study population, though the definition of a sudden loss was not well defined.
Patients without a tumor were more likely to have “flat” audiograms (P < .05), “trough” audiograms (P < .05) and “reverse slope” audiograms (P < .01). In general, the latter suggests that there is more low frequency hearing loss in nontumor patients.
Asymmetric tinnitus was seen equally in tumor patients (60%) versus nontumor patients (64%).
A mean of 39% discrimination loss was seen in VS ears vs 23% loss in nontumor ears (P < .01). The mean intra-aural difference was significantly greater in tumor patients (mean 35% difference) vs controls (19% difference, P < .0001). The discrimination loss was less for tumors <11 mm (31% mean) than for larger tumors (47% mean).
Conclusions: The most sensitive screening test for tumor diagnosis in the study population was either: 1) asymmetry >15 dB at any frequency (0.5–4 kHz) or 2) asymmetry >19 dB at 2 contiguous frequencies. The most specific test for tumor diagnosis in the study population was: males: average asymmetry >19 dB (1–8 kHz); females: asymmetry at 4 kHz >19 dB.
|
|
Saliba et al, 2009
|
Retrospective review from a single center that attempted to analyze symptoms most predictive of a VS diagnosis on MRI using patients seen between 2003–2007. To be included, patients had to have had an audiogram, an ENG, and a contrast-enhanced MRI. Hearing asymmetry was defined as 15 dB at ≥1 frequencies or 15% or more discrimination asymmetry.
|
III
|
Results: 74 of 115 (64%) patients meeting these criteria had a VS.
59/74 (80%) tumor patients had asymmetric tinnitus, and 32/48 (67%) nontumor patients had asymmetric tinnitus (P = .078).
25 nontumor patients had vertigo vs 9 tumor patients (P < .001). However, the vestibular deficit percentage was 45% for the tumor patients and 25% for the nontumor patients (P = .049)
Conclusion: Tinnitus and vestibular handicap alone are not reliable predictors of a VS diagnosis. Pure tone asymmetry of ≥15 dB at 3000 Hz should be considered as a VS screening tool because of its relatively high sensitivity and specificity.
|
|
Cadoni et al, 2006
|
Retrospective review of patients presenting with a SSNHL from a single center (no date range provided).
SSNHL was defined as a difference of 30 dB at 3 contiguous frequencies over 3 days or less.
|
III
|
Results: 54 patients who met these criteria were screened with a contrast-enhanced MRI.
In this series, an MRI abnormality within the auditory pathway was found in 11% of cases of SSNHL, but only 1 VS was identified. Other lesions identified included cochlear inflammation, arachnoid cyst, demyelination, and a pontine lesion.
Conclusion: SSNHL is a rare presenting sign of a VS, although this clinical finding should not be discounted when it occurs. On the basis of these findings, the authors advocate for an MRI screen in patients with a SSNHL.
|
|
Sauvaget et al, 2005
|
Retrospective review of patients from a single center between 2000–2002. VS diagnosis was proven on the basis of histopathology. The definition of a sudden hearing loss was not provided.
|
III
|
Results: 139 patients with tumors were analyzed. 23 cases from the study group experienced a single sudden hearing loss episode before their tumor diagnosis, and 5 patients had >1 episode of sudden hearing loss before diagnosis. However, these numbers reflect a patient’s subjective report of sudden hearing loss. Only 21 total patients had their hearing loss verified with an audiogram.
Conclusion: SSNHL may be more common than is generally appreciated, which is attributable to the belief that not all patients who experience a sudden loss seek medical attention or undergo an audiogram. On the basis of these findings, the authors advocate for MRI screening of patients with a sudden hearing loss.
|
|
Aarnisalo et al, 2004
|
Planned case review of SSNHL cases between 1999–2000 at a single center. Sudden hearing loss was defined as a 25-dB average difference at 3 contiguous frequencies occurring in ≤3 weeks.
|
III
|
Results: Using this definition, 30 cases were found, 82 of which had screening MRIs with gadolinium contrast. Of 82 study patients with an MRI, 29 patients had identification of a definite abnormality. 12 of these patients had a pathology that was possibly related to the hearing loss. 4 of these patients had a VS.
Conclusion: Performing an MRI shortly after a SSNHL can be helpful to establish a diagnosis. On the basis of these findings, the authors advocate for MRI screening of patients with a SSNHL.
|
|
Nageris et al, 2003
|
Retrospective review of a single center’s experience with SSNHL between 1989–2000. Sudden hearing loss was defined as any patient who presented with a 10-dB loss in ≥2 frequencies over a poorly defined “few days.” These patients were screened with a contrast-enhanced MRI. Patients were excluded if they were ultimately diagnosed with Meniere disease, a perilymphatic fistula, middle or external ear disease, or “systemic” disease.
|
III
|
Results: The study criteria identified 67 patients with asymmetric SSNHL. 24 patients had VSs, while 43 did not. In the study population, 16.7% of tumor patients had a complete hearing recovery. The authors make the point that when hearing recovery occurs after a SSNHL, it does not rule out the possibility of a VS.
Conclusion: SSNHL can be a presenting sign of a VS, and hearing can recover in these patients, although recovery is rare. Based on these results, the authors advocate for MRI screening of patients with a SSNHL, even if recovery is demonstrated.
|
|
Haapaniemi et al, 2000
|
Retrospective review of patients diagnosed with a VS at a single center between 1992–1997. Diagnosis was made using contrast-enhanced MRI. The authors sought to evaluate the symptoms of patients presenting with a tumor and to correlate these symptoms with tumor size and location. SSNHL, asymmetric hearing loss, dizziness, and tinnitus were evaluated. No clear definition was given for sudden hearing loss or asymmetric hearing loss.
|
III
|
Results: 41 total patients were analyzed. Out of the study patients, 5 patients experienced a sudden hearing loss, and 4 patients experienced asymmetric tinnitus as their chief complaints. 5 patients experienced subjective dizziness as their chief complaint.
Conclusion: Both sudden hearing loss and asymmetric tinnitus are rare initial symptoms in patients diagnosed with a VS when compared with the patients who present with an initial complaint of asymmetric hearing loss. The authors recommend MRI screening for any patient experiencing these symptoms.
|
|
Magdziarz et al, 2000
|
Multicenter, retrospective review performed between 1980–1997 to evaluate patients that present with relatively “normal” audiologic findings and are ultimately diagnosed with a VS.
To be included, patients had to have comprehensive audiometry, ABR testing, and surgical histopathology for the tumor after resection.
To be “normal,” audiometric findings had to include speech discrimination >90% in the bad ear with a pure tone intra-aural difference of <10 dB at any one frequency for 500, 1000, and 2000 Hz.
|
III
|
Results: 369 VS patients were identified, of which 10 had relatively “normal” hearing. Moreover, once these 10 patients were identified, a matched comparison was done with 10 patients who had tumors and hearing loss. A match was made on the basis of:
1) Age within 5 years
2) Tumor size within 0.3 cm
3) Tumor location (classified as IAC ± CPA OR CPA ± IAC)
4) ABR findings preoperatively
In the 10 patients with “normal” hearing, the mean age was 39.1 years (range 29–49 years). As per the definition, 0% had a SDS <90% in the tumor ear. No patient was found to have audiometric rollover.
Out of the 359 tumor patients with “abnormal hearing” the mean age was 50.2 years (range 10–86 years). 86% had SDS <90% in the tumor ear. 55.2% had audiometric rollover.
In the 10 matched, control patients with “abnormal” hearing, 70% had SDS <90% in the tumor ear.
2.7% of 369 patients with proven tumors presented with “normal” hearing. Disequilibrium, asymmetric tinnitus (4/10 patients), and vertigo were the most common symptoms in the “normal” hearing group. Average tumor size was smaller in patients with “normal” hearing (1.44 vs 1.96 cm), although statistical analysis was not clearly performed.
Conclusion: Tumors can be present in the absence of pure tone asymmetry as measured on audiometry. On the basis of their results, the authors conclude that unexplained symptoms, particularly those relating to balance and tinnitus, should be evaluated with an MRI given the possibility that tumors may be present in the setting of relatively “normal” audiometric findings.
|
|
Dawes et al, 1999
|
Retrospective review of patients sent to a single center for a screening MRI from 1994–1997. Unilateral tinnitus was the primary concern for screening.
|
III
|
Results: 174 patients received a contrast-enhanced MRI for unilateral tinnitus. In this study, 1 patient of 174 (0.6%) had a tumor found after screening for this reason. However, there were 18 other patients who had “positive” findings on the MRI that merited further investigation. In total, approximately 3.4% of the study population had a finding on MRI that was considered to be causative for the tinnitus.
Conclusion: Unilateral tinnitus is a rare presentation of a VS in the absence of an asymmetric hearing loss. The authors suggest that the findings presented in this study justify MRI screening for asymmetric tinnitus.
|
|
Fitzgerald et al, 1998
|
Retrospective review from a single center of SSNHL cases between 1989–1995 screened with a contrast-enhanced MRI. Sudden hearing loss was defined as a >30 dB decrease in thresholds in ≥3 contiguous test frequencies occurring over a 24- to 72-hour period.
|
III
|
Results: 78 consecutive patients were identified who met these criteria. 31% of patients had abnormal findings on the MRI that were presumed to be the cause of the sudden hearing loss. The frequency of VS identification was 4%.
Conclusion: Asymmetric SSNHL is not infrequently associated with a recognizable pathology on MRI. On the basis of these findings, the authors advocate an MRI when SSNHL is identified on audiogram.
|
|
Lustig et al, 1998
|
Retrospective review of all patients diagnosed with VSs at a single center between 1983–1996 in order to identify VS patients who presented with symmetric hearing on audiogram. The definition of symmetric hearing was an interaural difference <15 dB at a single frequency or <10 dB at 2 or more frequencies (500, 1K, 2K and 4K Hz). Symmetry also required a SRT <20 dB and an interaural speech discrimination score differential of <20%.
|
III
|
Results: In total, 29 “normal hearing” patients were identified out of 546 VS patients. 9 patients were male, and 20 were female. In this study, 5.3% of VS patients met their definition of symmetric acoustic thresholds. Amongst this group, the most common indications for MRI diagnosis were subjective disequilibrium (41%), cranial nerve abnormalities (38%), NF2 family screening (17%), and asymmetric tinnitus (14%), subjective hearing loss (14%), headache (14%) and incidental finding. In one case of symmetric hearing thresholds, a 3.5-cm tumor was identified.
Conclusion: Tumors can be present in the absence of pure tone asymmetry as measured on audiometry. Due to the possibility of tumor patients presenting with symmetric hearing, the authors recommend that persistent vestibulocochlear complaints be evaluated.
|
|
Levy et al, 1996
|
Retrospective analysis from a single center over 2 years (no dates given) to analyze the MRI findings in patients with vestibulocochlear dysfunction. Patients were generally screened with an MRI to evaluate vestibular symptoms, abnormal ABR findings, or abnormal audiogram findings. Audiometric findings considered to be abnormal were asymmetric hearing loss of ≥25 dB at 2 or more frequencies between 1–8 kHz or ≥20% asymmetry in discrimination.
|
III
|
Results: In total, 118 patients were screened. Of 118 patients, 9 were found to have a definite VS based on MRI or pathology. 5 of these 9 patients had asymmetric hearing and 6 of the 9 patients had asymmetric tinnitus. Therefore, there was 1 patient who presented with asymmetric tinnitus in the absence of an asymmetric hearing loss.
Conclusion: The authors in this study conclude that there are no symptoms or audiometric findings that are clearly sensitive for VS diagnosis.
|
|
Neary et al, 1996
|
Retrospective review of patients with a proven VS diagnosis from a single center between 1991–1994. Audiologic findings were analyzed. Patients were included for analysis if there was a proven histologic diagnosis (80) or a contrast-enhanced MRI confirmed diagnosis (13), leading to a total of 93 patients. There was no clear definition of asymmetric hearing loss on the basis of an audiogram.
|
III
|
Results: 93 patients were identified. The mean age of symptom onset was 44.4 years. Out of the 93 patients, 44 patients presented with asymmetric SNHL, while 14 patients presented with simply unilateral tinnitus. 7 patients had SSNHL, which was not clearly defined in audiometric terms.
Conclusion: The authors of this study emphasized the importance of a thorough history and physical to identify signs and symptoms suggestive of a tumor diagnosis. MRI with gadolinium contrast was also their method of choice for diagnosis, citing the insensitivity of other audiometric testing.
|
|
Van Leeuwen et al, 1995
|
Retrospective review of patients from a single center who had a pathologically proven VS between 1980–1992 in order to analyze patient symptom presentation.
|
III
|
Results: 164 tumors patients were analyzed including 88 women and 76 men. The mean age at diagnosis was 49.2 years (range 17–79 years), and the mean tumor size was 26.5 mm (range 8–72 mm). Of 164 tumors, 93% had asymmetric hearing, but no clear audiometric data were given to quantify this statistic. Pure tone data was not clearly presented, and the definition of “asymmetric” was not provided. However, 57% of patients had asymmetric tinnitus, and 3% had sudden deafness, subjectively. It was unclear whether or not all patients with sudden deafness had a preoperative audiogram. An assessment between tumor size and symptoms was made, in which no clear correlation was found.
Conclusion: The authors concluded that most patients have asymmetric hearing loss as a presenting symptom, and tumor size does not correlate with symptoms.
|
|
Saunders et al, 1995
|
Retrospective review from a single center including patients from 1982–1993 in order to evaluate patients with SSNHL. Patients who had a documented sudden hearing loss of 25 dB at ≥1 frequencies over 48 hours or less (836), and patients who were diagnosed with a VS (1487) were analyzed separately.
|
III
|
Results: Only 431 patients with a SSNHL were screened with a contrast-enhanced MRI to prove the diagnosis, and 1204 cases of the 1487 with a VS had reliable descriptions of their presenting symptoms. In total, 13 of 431 (3%) patients with SSNHL were found to have a VS on MRI. A sudden hearing loss was documented in 79 of the 1204 (7%) VS patients. Furthermore, it was reported that 15.4% of patients had an asymmetric tinnitus prior to experiencing a sudden hearing loss, although it was unclear how these patients were screened or how the tumor was diagnosed.
Conclusion: Based on these data, the authors conclude that SSNHL should be evaluated further with additional diagnostic studies. The authors also suggest that facial pain, paresthesia, and asymmetric tinnitus can be suggestive of a tumor diagnosis.
|
Table 3. Comparison of asymmetric sensorineural hearing loss criteria
|
Lead Author, Date
|
No. of Patients Screened
|
No. of Tumors Identified
|
Most Sensitive
|
Most Specific
|
|
Saliba et al, 2011
|
212
|
84
|
≥10 dB at 2 contiguous frequencies OR 15 dB at any single frequency (0.93)
|
≥15 dB difference at 3000 Hz (0.76)
|
|
Gimsing, 2010
|
428
|
203
|
≥15 dB at any frequency OR 20 dB at 2 contiguous frequencies (0.93)
|
Males: average asymmetry
≥20 dB (1–8 kHz); females: asymmetry ≥20 dB at 4 kHz
|
Table 4. Articles in which asymmetric tinnitus was a presenting symptom used for VS screening
|
Lead Author, Date
|
Patients with Asymmetric Tinnitus
|
Patients Diagnosed with a VS
|
|
Dawes et al, 1999
|
174
|
1
|
|
Lustig et al, 1998
|
546
|
4
|
Table 5. Articles in which asymmetric tinnitus was noted by VS patients at the time of presentation, irrespective of other symptoms
|
Lead Author, Date
|
Patients with Asymmetric Tinnitus
|
Patients Diagnosed with a VS
|
Comparison Group
|
|
Gimsing, 2010
|
122
|
203
|
144 of 225 patients with an asymmetric hearing loss but without a VS also had asymmetric tinnitus
|
|
Saliba et al, 2009
|
59
|
74
|
32 of 48 patients with an asymmetric hearing loss but without a VS also had asymmetric tinnitus
|
|
Haapaniemi et al, 2000
|
25
|
41
|
None
|
|
Neary et al, 1996
|
14
|
93
|
None
|
|
Levy et al, 1996
|
6
|
9
|
None
|
|
Van Leeuween et al, 1995
|
93
|
164
|
None
|
Table 6. Articles in which SSNHL was a specific finding used for VS screening
|
Lead Author, Date
|
Patients with SSNHL
|
Patients Diagnosed with VS
|
|
Lee et al, 2011
|
295
|
9
|
|
Cadoni et al, 2006
|
54
|
1
|
|
Aarnisalo et al, 2004
|
82
|
4
|
|
Nageris et al, 2003
|
67
|
24
|
|
Fitzgerald et al, 1998
|
78
|
3
|
|
Saunders et al, 1995*
|
431
|
13
|
*Patient numbers are based on the number meeting inclusion criteria from the present study.
Table 7. Articles in which SSNHL was noted by VS Patients at the time of presentation, irrespective of other symptoms
|
Lead Author, Date
|
Patients with SSNHL
|
Patients diagnosed with VS
|
|
Gimsing, 2010
|
21
|
203
|
|
Sauvaget et al, 2005*
|
21
|
139
|
|
Haapaniemi et al, 2000
|
5
|
41
|
|
Neary et al, 1996
|
7
|
93
|
|
Saunders et al, 1995
|
79
|
1204
|
*Patient numbers are based on the number meeting inclusion criteria from the present study.
References
1. Cueva RA. Auditory brainstem response versus magnetic resonance imaging for the evaluation of asymmetric sensorineural hearing loss. Laryngoscope 2004;114(10):1686-1692.
2. Stangerup SE, Tos M, Thomsen J, Caye-Thomasen P. True incidence of vestibular schwannoma? Neurosurgery 2010;67(5):1335-1340; discussion 1340.
3. Schonberg MA, Breslau ES, McCarthy EP. Targeting of mammography screening according to life expectancy in women aged 75 and older. J Am Geriatr Soc 2013;61(3):388-395.
4. Schonberg MA, Breslau ES, Hamel MB, Bellizzi KM, McCarthy EP. Colon cancer screening in U.S. adults aged 65 and older according to life expectancy and age. J Am Geriatr Soc 2015;63(4):750-756.
5. Khrais T, Romano G, Sanna M. Nerve origin of vestibular schwannoma: a prospective study. J Laryngol Otol 2008;122(2):128-131.
6. Xenellis JE, Linthicum Jr FH. On the myth of the glial/schwann junction (Obersteiner-Redlich zone): origin of vestibular nerve schwannomas. Otol Neurotol 2003;24(1):1.
7. Lustig LR, Rifkin S, Jackler RK, Pitts LH. Acoustic neuromas presenting with normal or symmetrical hearing: factors associated with diagnosis and outcome. Am J Otol 1998;19(2):212-218.
8. Cheng TC, Wareing MJ. Three-year ear, nose, and throat cross-sectional analysis of audiometric protocols for magnetic resonance imaging screening of acoustic tumors. Otolaryngol Head Neck Surg 2012;146(3):438-447.
9. Saliba I, Martineau G, Chagnon M. Asymmetric hearing loss: rule 3,000 for screening vestibular schwannoma. Otol Neurotol 2009;30(4):515-521.
10. Saliba I, Bergeron M, Martineau G, Chagnon M. Rule 3,000: a more reliable precursor to perceive vestibular schwannoma on MRI in screened asymmetric sensorineural hearing loss. Eur Arch Otorhinolaryngol 2011;268(2):207-212.
11. Gimsing S. Vestibular schwannoma: when to look for it? J Laryngol Otol 2010;124(3):258-264.
12. Nageris BI, Popovtzer A. Acoustic neuroma in patients with completely resolved sudden hearing loss. Ann Otol Rhinol Laryngol 2003;112(5):395-397.
13. Magdziarz DD, Wiet RJ, Dinces EA, Adamiec LC. Normal audiologic presentations in patients with acoustic neuroma: an evaluation using strict audiologic parameters. Otolaryngol Head Neck Surg 2000;122(2):157-162.
14. Dawes PJ, Basiouny HE. Outcome of using magnetic resonance imaging as an initial screen to exclude vestibular schwannoma in patients presenting with unilateral tinnitus. J Laryngol Otol 1999;113(9):818-822.
15. Levy RA, Arts HA. Predicting neuroradiologic outcome in patients referred for audiovestibular dysfunction. AJNR Am J Neuroradiol 1996;17(9):1717-1724.
16. van Leeuwen JP, Cremers CW, Thewissen NP, Harhangi BS, Meijer E. Acoustic neuroma: correlation among tumor size, symptoms, and patient age. Laryngoscope 1995;105(7 pt 1):701-707.
17. Saunders JE, Luxford WM, Devgan KK, Fetterman BL. Sudden hearing loss in acoustic neuroma patients. Otolaryngol Head Neck Surg 1995;113(1):23-31.
18. Fitzgerald DC, Mark AS. Sudden hearing loss: frequency of abnormal findings on contrast-enhanced MR studies. AJNR Am J Neuroradiol 1998;19(8):1433-1436.
19. Aarnisalo AA, Suoranta H, Ylikoski J. Magnetic resonance imaging findings in the auditory pathway of patients with sudden deafness. Otol Neurotol 2004;25(3):245-249.
20. Lee JD, Lee BD, Hwang SC. Vestibular schwannoma in patients with sudden sensorineural hearing loss. Skull Base 2011;21(2):75-78.
21. Cadoni G, Cianfoni A, Agostino S, et al. Magnetic resonance imaging findings in sudden sensorineural hearing loss. J Otolaryngol 2006;35(5):310-316.
22. Haapaniemi J, Laurikainen E, Johansson R, Miettinen S, Varpula M. Cochleovestibular symptoms related to the site of vestibular schwannoma. Acta Otolaryngol Suppl 2000;543:14-16.
23. Sauvaget E, Kici S, Kania R, Herman P, Tran Ba Huy P. Sudden sensorineural hearing loss as a revealing symptom of vestibular schwannoma. Acta Otolaryngol 2005;125(6):592-595.
24. Neary WJ, Newton VE, Laoide-Kemp SN, Ramsden RT, Hillier VF, Kan SW. A clinical, genetic and audiological study of patients and families with unilateral vestibular schwannomas. II. Audiological findings in 93 patients with unilateral vestibular schwannomas. J Laryngol Otol 1996;110(12):1120-1128.
25. Kwan TL, Tang KW, Pak KK, Cheung JY. Screening for vestibular schwannoma by magnetic resonance imaging: analysis of 1821 patients. Hong Kong Med J 2004;10(1):38-43.
26. Daniels RL, Swallow C, Shelton C, Davidson HC, Krejci CS, Harnsberger HR. Causes of unilateral sensorineural hearing loss screened by high-resolution fast spin echo magnetic resonance imaging: review of 1,070 consecutive cases. Am J Otol 2000;21(2):173-180.
27. Schick B, Brors D, Koch O, Schafers M, Kahle G. Magnetic resonance imaging in patients with sudden hearing loss, tinnitus and vertigo. Otol Neurotol 2001;22(6):808-812.
28. Newton JR, Shakeel M, Flatman S, Beattie C, Ram B. Magnetic resonance imaging screening in acoustic neuroma. Am J Otolaryngol 2010;31(4):217-220.
29. Chisholm EJ, Savy L, Geyer M, Choa D. Magnetic resonance imaging scans for vestibulocochlear nerve tumours: what is actually found? J Laryngol Otol 2006;120(12):1019-1022.
30. Mirza S, Malik TH, Ahmed A, Willatt DJ, Hughes DG. Incidental findings on magnetic resonance imaging screening for cerebellopontine angle tumours. J Laryngol Otol 2000;114(10):750-754.
31. Dawes PJ, Jeannon JP. Audit of regional screening guidelines for vestibular schwannoma. J Laryngol Otol 1998;112(9):860-864.
32. Shargorodsky J, Curhan SG, Curhan GC, Eavey R. Change in prevalence of hearing loss in US adolescents. JAMA 2010;304(7):772-778.
33. Nageris BI, Raveh E, Zilberberg M, Attias J. Asymmetry in noise-induced hearing loss: relevance of acoustic reflex and left or right handedness. Otol Neurotol 2007;28(4):434-437.
34. Berg RL, Pickett W, Linneman JG, Wood DJ, Marlenga B. Asymmetry in noise-induced hearing loss: evaluation of two competing theories. Noise Health 2014;16(69):102-107.
35. Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg 2014;151(2 suppl):S1-S40.
36. Hinkley LB, Mizuiri D, Hong O, Nagarajan SS, Cheung SW. Increased striatal functional connectivity with auditory cortex in tinnitus. Front Hum Neurosci 2015;9:568.
37. Larson PS, Cheung SW. Deep brain stimulation in area LC controllably triggers auditory phantom percepts. Neurosurgery 2012;70(2):398-405.
38. Acoustic Neuroma Association website. 2014 report on ANA patient database. Available at: https://www.anausa.org/images/pdf/Patient-Survey/Patient_Survey_Report_F.... Accessed November 22, 2017.
39. Overdevest JB, Pross SE, Cheung SW. Tinnitus following treatment for sporadic acoustic neuroma. Laryngoscope 25 2015.
40. Cushing H. Tumors of the Nervus Acusticus and the Syndrome of the Cerebellopontine Angle. Philadelphia, PA: W.B. Saunders; 1917.
41. Higgs WA. Sudden deafness as the presenting symptom of acoustic neurinoma. Arch Otolaryngol 1973;98(2):73-76.
42. Miller ME, Mafee MF, Bykowski J, et al. Hearing preservation and vestibular schwannoma: intracochlear FLAIR signal relates to hearing level. Otol Neurotol 2014;35(2):348-352.
43. Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg 2012;146(3 suppl):S1-35.
44. Ruckenstein MJ, Cueva RA, Morrison DH, Press G. A prospective study of ABR and MRI in the screening for vestibular schwannomas. Am J Otol 1996;17(2):317-320.
45. Shelton C, Harnsberger HR, Allen R, King B. Fast spin echo magnetic resonance imaging: clinical application in screening for acoustic neuroma. Otolaryngol Head Neck Surg 1996;114(1):71-76.
46. Annesley-Williams DJ, Laitt RD, Jenkins JP, Ramsden RT, Gillespie JE. Magnetic resonance imaging in the investigation of sensorineural hearing loss: is contrast enhancement still necessary? J Laryngol Otol 2001;115(1):14-21.
47. Murphy MR, Selesnick SH. Cost-effective diagnosis of acoustic neuromas: a philosophical, macroeconomic, and technological decision. Otolaryngol Head Neck Surg 2002;127(4):253-259.
48. Ryan M, Weissman JL, Kaylie D. Is Gadolinium contrast enhancement necessary in screening MRI for asymmetric sensorineural hearing loss? Laryngoscope 2015;125(4):783-784.
49. Rosenberg SI. Natural history of acoustic neuromas. Laryngoscope 2000;110(4):497-508.
50. Stangerup SE, Caye-Thomasen P, Tos M, Thomsen J. The natural history of vestibular schwannoma. Otol Neurotol 2006;27(4):547-552.
51. Bakkouri WE, Kania RE, Guichard JP, Lot G, Herman P, Huy PT. Conservative management of 386 cases of unilateral vestibular schwannoma: tumor growth and consequences for treatment. J Neurosurg 2009;110(4):662-669.
52. Varughese JK, Breivik CN, Wentzel-Larsen T, Lund-Johansen M. Growth of untreated vestibular schwannoma: a prospective study. J Neurosurg 2012;116(4):706-712.
53. Fayad JN, Semaan MT, Lin J, Berliner KI, Brackmann DE. Conservative management of vestibular schwannoma: expectations based on the length of the observation period. Otol Neurotol 2014;35(7):1258-1265.
54. Moffat DA, Baguley DM, Beynon GJ, Da Cruz M. Clinical acumen and vestibular schwannoma. Am J Otol 1998;19(1):82-87.
© Congress of Neurological Surgeons
Source: Neurosurgery, February 2018