Guidelines on the Management of Patients with Vestibular Schwannoma
8. Surgical Resection for the Treatment of Patients with Vestibular Schwannomas
download pdf Neurosurgery, 2017
Sponsored by: Congress of Neurological Surgeons (CNS) and the AANS/CNS Tumor Section
Endorsed by: Joint Guidelines Committee of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS)
Authors:
Constantinos G. Hadjipanayis, MD, PhD1, Matthew L. Carlson, MD2,3, Michael J. Link, MD3, Tarek A. Rayan, MD, PhD3, John Parish, MD4, Tyler Atkins, MD4, Anthony L. Asher, MD5, Ian F. Dunn, MD6, C. Eduardo Corrales, MD7, Jamie J. Van Gompel, MD2,3, Michael Sughrue, MD8, Jeffrey J. Olson, MD9
1.Department of Neurosurgery, Mount Sinai Beth Israel, Icahn School of Medicine at Mount Sinai, New York, New York, USA
2.Department of Otorhinolaryngology, Mayo Clinic School of Medicine, Rochester, Minnesota, USA
3.Department of Neurologic Surgery, Mayo Clinic, Rochester, Minnesota, USA
4. Carolinas Medical Center, Charlotte, North Carolina, USA
5. Carolina Neurosurgery & Spine Associates, Charlotte, North Carolina
6.Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA
7.Division of Otolaryngology-Head and Neck Surgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA
8.Department of Neurosurgery, University of Oklahoma, Oklahoma City, Oklahoma, USA
9.Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia, USA
Correspondence:
Constantinos G. Hadjipanayis, MD, PhD
Department of Neurosurgery
Mount Sinai Downtown Union Square
10 Union Square East, 5th Floor, Suite 5E
New York, New York 10580
Telephone: 212-844-6922; Fax: 212-844-6119
Email: Constantinos.Hadjipanayis@mountsinai.org
Keywords: Acoustic neuroma, Neurofibromatosis type 2, stereotactic radiosurgery, surgical resection, vestibular schwannoma
Abbreviations
AAO-HNS: American Academy of Otolaryngology-Head and Neck Surgery
AN: Acoustic neuroma
CPA: Cerebellopontine angle
DHI: Dizziness handicap index
FN: Facial nerve
FSRT: Fractionated stereotactic radiotherapy
GBI: Glasgow Benefit Inventory
GKRS: Gamma Knife radiosurgery
GTR: Gross total resection
HB: House–Brackmann
HP: Hearing preservation
IAC: Internal auditory canal
IC: Intracanalicular
IOM: Intraoperative monitoring
MF: Middle fossa
MPNST: Malignant peripheral nerve sheath tumor
NF2: Neurofibromatosis type 2
NTR: Near total resection
QOI: Quality of life
RS: Retrosigmoid
SDS: Speech discrimination score
SRS: Stereotactic radiosurgery
STR: Subtotal resection
TL: Translabyrinthine
VS: Vestibular schwannoma
No part of this manuscript has been published or submitted for publication elsewhere.
Abstract
Question 1
What surgical approaches for vestibular schwannomas (VS) are best for complete resection and facial nerve (FN) preservation when serviceable hearing is present?
Target Population
These recommendations apply to adults with sporadic VSs who underwent microsurgical resection via the retrosigmoid (RS) or middle fossa (MF) approach.
Recommendation
There is insufficient evidence to support superiority of either the MF or RS approach for complete VS resection and FN preservation when serviceable hearing is present.
Question 2
Which surgical approach (RS or translabyrinthine [TL]) for VSs is best for complete resection and FN preservation when serviceable hearing is not present?
Target Population
This recommendation applies to adults with sporadic VSs who underwent microsurgical resection via the RS or TL approach.
Recommendation
There is insufficient evidence to support superiority of either the RS or TL approach for complete VS resection and FN preservation when serviceable hearing is not present.
Question 3
Does VS size matter for facial and vestibulocochlear nerve preservation with surgical resection?
Target Population
This recommendation applies to adults with sporadic VSs who underwent microsurgical resection via the TL, RS, or MF approach.
Recommendation
Level 3: Patients with larger VS tumor size should be counseled about the greater than average risk of loss of serviceable hearing.
Question 4
Should small intracanalicular tumors (< 1.5 cm) be surgically resected?
Target Population
This recommendation applies to adults with sporadic VSs who underwent microsurgical resection.
Recommendation
There are insufficient data to support a firm recommendation that surgery be the primary treatment for this subclass of VSs.
Question 5
Is hearing preservation routinely possible with VS surgical resection when serviceable hearing is present?
Target Population
This recommendation applies to adults with both sporadic and Neurofibromatosis type 2 (NF2) VSs undergoing microsurgical resection via the MF or RS approach.
Recommendation
Level 3: Hearing preservation surgery via the MF or the RS approach may be attempted in patients with small tumor size (< 1.5 cm) and good preoperative hearing.
Question 6
When should surgical resection be the initial treatment in patients with NF2?
Target Population
This recommendation applies to patients meeting diagnostic criteria for NF2.
Recommendation
There is insufficient evidence that surgical resection should be the initial treatment in patients with NF2.
Question 7
Does a multidisciplinary team, consisting of neurosurgery and neurotology, provide the best outcomes of complete resection and facial/vestibulocochlear nerve preservation for patients undergoing resection of VSs?
Target Population
This recommendation applies to adults with sporadic VSs who underwent microsurgical resection.
Recommendation
There is insufficient evidence to support stating that a multidisciplinary team, usually consisting of a neurosurgeon and a neurotologist, provides superior outcomes compared to either subspecialist working alone.
Question 8
Does a subtotal surgical resection of a VS followed by stereotactic radiosurgery (SRS) to the residual tumor provide comparable hearing and FN preservation to patients who undergo a complete surgical resection?
Target Population
This recommendation applies to adults with sporadic VSs who underwent microsurgical resection.
Recommendation
There is insufficient evidence to support subtotal resection followed by SRS provides comparable hearing and FN preservation to patients who undergo a complete surgical resection.
Question 9
Does surgical resection of VSs treat preoperative balance problems more effectively than SRS?
Target Population
This recommendation applies to adults with sporadic VSs who underwent microsurgical resection or SRS treatment.
Recommendation
There is insufficient evidence to support either surgical resection or SRS for treatment of preoperative balance problems.
Question 10
Does surgical resection of VSs treat preoperative trigeminal neuralgia more effectively than SRS?
Target Population
This recommendation applies to adults with sporadic VSs who underwent microsurgical resection or SRS treatment.
Recommendation
Level 3: Surgical resection of VSs may be used to better relieve symptoms of trigeminal neuralgia than SRS.
Question 11
Is surgical resection of VSs more difficult (associated with higher facial neuropathies and subtotal resection rates) after initial treatment with SRS?
Target Population
This recommendation applies to adults with sporadic VSs who underwent microsurgical resection after SRS treatment.
Recommendation
Level 3: If microsurgical resection is necessary after SRS, it is recommended that patients be counseled that there is an increased likelihood of a subtotal resection and decreased FN function.
Introduction
Vestibular schwannomas (VSs) are slow-growing, benign tumors that typically arise from the vestibular portion of the eighth cranial nerve. More than 95% of VSs are sporadic in nature, while approximately 5% are associated with neurofibromatosis type 2 (NF2), an autosomal dominant syndrome hallmarked by the development of bilateral VSs.1
Through the introduction of subcapsular subtotal tumor resection and the use of electrocautery, Harvey Cushing was able to reduce the mortality rate of VS surgery to approximately 20%. Subsequently, Walter Dandy advocated a unilateral retrosigmoid (RS) suboccipital approach with gross total tumor removal. The next major advance came with the adoption of the operating microscope and a revival of the translabyrinthine (TL) and middle fossa (MF) approaches by William House. Further refinements in cranial nerve monitoring and microsurgical technique have offered the opportunity for facial nerve (FN) preservation in the great majority of cases and hearing preservation in select patients. Despite advances in radiation delivery and an improved understanding of the natural history of VS growth, microsurgical resection continues to be the most common treatment option used in the United States, and it remains the preferred modality for young patients, large (>3 cm) VSs, cystic tumors, or VSs that result in symptoms of mass effect. The surgical treatment of VSs is highly nuanced and varies between institutions. The following systematic review was performed to provide a set of evidence-based guidelines for the surgical management of VSs.
Rationale
Complete tumor removal and cranial nerve preservation are the goals of any VS surgical resection. The success of surgical resection of VSs may be impacted by the surgical approach and serviceable hearing status of the patient, tumor size and location, NF2 status, multidisciplinary team management, combination treatment with SRS, previous SRS treatment, and preoperative symptoms.
Objectives
The objectives of this guideline are to assess both comparative and noncomparative studies of surgical management of VSs based on the following questions:
- What surgical approaches for VSs are best for complete resection and facial nerve preservation when serviceable hearing is present?
- What surgical approaches for VSs are best for complete resection and facial nerve preservation when serviceable hearing is not present?
- Does VS size matter for facial and vestibulocochlear nerve preservation with surgical resection?
- Should small intracanalicular tumors (< 1.5 cm) be surgically resected?
- Is hearing preservation routinely possible with VS surgical resection?
- When should surgical resection be the primary treatment in patients with NF2?
- Does a multidisciplinary team, consisting of neurosurgery and neurotology, provide the best outcomes of complete resection and facial/vestibulocochlear nerve preservation for patients undergoing resection of VSs?
- Does a subtotal surgical resection of a VS followed by radiosurgery to the residual tumor provide comparable outcomes in patients who undergo a complete surgical resection?
- Does surgical resection of VSs treat preoperative balance problems more effectively than stereotactic radiosurgery (SRS)?
- Does surgical resection of VSs treat preoperative trigeminal neuralgia more effectively than SRS?
- Is surgical resection of VSs more difficult (associated with higher facial neuropathies and subtotal resection rates) after initial treatment with SRS?
Methods
Writing Group and Question Establishment
The evidence-based clinical practice guideline taskforce members and the Joint Tumor Section of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS) have prioritized writing the guidelines for management of VSs. A series of authors for the development of guidelines related to surgical management of VSs were identified and screened for conflict of interest. This group in turn agreed on a set of questions addressing the topic at hand and conducted a systematic review of the literature relevant to the surgical management of VSs. Additional details of the systematic review are provided below and within the introduction and methodology chapter of the guideline (here).
Search Method
The task force collaborated with a medical librarian to search for articles published from January 1990 through 2014. Two electronic databases, PubMed and the Cochrane Central Register of Controlled Trials (see below), were searched. Strategies for searching electronic databases were constructed by the Evidence-based clinical practice guideline taskforce members and the medical librarian using standard strategies to identify relevant studies.6–13
PubMed Search
- Neuroma, acoustic [MeSH]
- (Vestibular [Title/Abstract] OR vestibulocochlear [Title/Abstract] OR acoustic [Title/Abstract]) AND (neuroma* [Title/Abstract] OR neurilemmoma* [Title/Abstract] OR neurilemoma* [Title/Abstract] OR neurinoma* [Title/Abstract] OR tumor* [Title/Abstract] OR tumour* [Title/Abstract] OR schwannoma* [Title/Abstract])
- #1 OR #2
- Neurosurgical procedures [MeSH] OR otologic surgical procedures [MeSH] OR minimally invasive surgical procedures [MeSH] OR radiosurgery [MeSH] OR microsurgery [MeSH] OR surgery [SH] OR radiotherapy [SH]
- Resection [TIAB] OR microsurger* [TIAB] OR microsurgical [TIAB] OR surger*[tiab] OR surgical [tiab] OR operati* [tiab] OR endoscop* [TIAB]OR suboccipital [TIAB] OR translabyrinthine [TIAB] OR middle fossa [TIAB] OR retrosigmoid [TIAB] OR transcochlear [TIAB] OR presigmoid [TIAB] OR transpetrosal [TIAB] OR extracisternal [TIAB] OR radiosurg* [TIAB] OR gamma knife [TIAB] OR cyberknife [TIAB] OR linac [TIAB] OR brainlab [TIAB] OR proton beam [TIAB] OR stereotact* [TIAB] OR stereotaxi* [TIAB] OR SRS [TIAB]
- #4 OR #5
- #3 AND #6
- (Animal [MeSH] NOT human [MeSH]) OR cadaver [MeSH] OR cadaver* [Titl] OR comment [PT] OR letter [PT] OR editorial [PT] OR addresses [PT] OR news [PT] OR “newspaper article” [PT] OR case reports [PT]
- #7 NOT #8
- #9 AND English [Lang]
- #10 AND (“1946/01/01” [PDAT] : “2015/01/01” [PDAT])
Cochrane Central Search
- MeSH descriptor: [neuroma, acoustic] explode all trees
- ((vestibular or vestibulocochlear or acoustic) and (neuroma* or neurilemmoma* or neurilemoma* or neurinoma* or tumor* or schwannoma*)):ti,ab,kw
- #1 or #2
- MeSH descriptor: [neurosurgical procedures] explode all trees
- MeSH descriptor [otologic surgical procedures] explode all trees
- MeSH descriptor [minimally invasive surgical procedures] explode all trees
- mesh descriptor radiosurgery explode all trees
- MeSH descriptor microsurgery
- MeSH Surgery [SH]
- Radiotherapy [SH]
- #4 or #5 or #6 or #7 or #8 or #9 or #10
- (Resection or microsurger* or microsurgical or surger* or surgical or operati* or endoscop* or suboccipital or translabyrinthine or “middle fossa” or retrosigmoid or transcochlear or presigmoid or transpetrosal or extracisternal or radiosurg* or “gamma knife” or cyberknife or linac or brainlab or “proton beam” or stereotact* or stereotaxi* or SRS):ti,ab,kw
- #11 or #12
- #3 and #13
Publication dates 1946–2014
The authors supplemented the searches of electronic databases with manual screening of the bibliographies of all retrieved publications. The authors also searched the bibliographies of recent systematic reviews and other review articles for potentially relevant citations. All articles identified were subject to the study selection criteria listed below. As noted above, the guideline committee also examined lists of included and excluded studies for errors and omissions. The authors went to great lengths to obtain a complete set of relevant articles. Having a complete set ensured that this guideline is not based on a biased subset of articles.
Study Selection and Eligibility Criteria
A total of 2949 citations were manually reviewed by the team with specific inclusion and exclusion criteria as outlined below. Two independent reviewers evaluated and abstracted full-text data for each article, and the 2 sets of data were compared for agreement by a third party. Inconsistencies were re-reviewed, and disagreements were resolved by consensus. Citations that considered adult patients focusing on surgical treatment of VSs were considered. To be included in this guideline, an article must be a report of a study that:
- Investigated patients suspected of having VSs
- Patients ≥18 years of age
- Was of humans
- Published between January 1, 1990 and December 31, 2014
- Quantitatively presented results
- Was not an in vitro study (for novel molecular markers, in vitro studies were included on patient samples)
- Was not a biomechanical study
- Was not performed on cadavers
- Was published in English
- Was not a meeting abstract, editorial, letter, or commentary
- Studies may include mixed pathology; however, the data pertaining to VSs was abstractable from the paper
- Had >5 patients or patient samples
The authors did not include systematic reviews, guidelines, or meta-analyses conducted by others. These documents are developed using different inclusion criteria than those specified in this guideline. Therefore, they may include studies that do not meet the inclusion criteria specified above. These documents were recalled if their abstract suggested that they might address one of the recommendations, and their bibliographies were searched for additional studies.
Data Collection Process
The abstracts that met the selection criteria mentioned above were retrieved in full-text form. Each article’s adherence to the selection criteria was confirmed. To determine how the data could be classified, the information in the full-text articles were then evaluated to determine whether they were providing results of therapy or were more centered on diagnostic or prognostic information. Agreement on these assessments and on the salient points regarding the type of study design and objectives, and the conclusions and data classification was then reached by exchanging drafts and comments by e-mail. The information was then used for construction of the evidence tables.
Assessment for Risk of Bias
All the literature reviewed was class III evidence (ie, evidence from nonexperimental descriptive studies, such as comparative studies, correlation studies, and case-control studies). Because the data analyzed were all class III, bias could be present because of selective case choice for study and selective results reporting, lack or loss of information over time, the biases of the interpreting investigator in regard to the study, publication bias regarding positive studies or positive cases, misclassification, survivorship bias, publication bias, recognition that data collected in this retrospective or prospective manner does not imply causation, selection bias, attrition bias, change in methods over time, ascertainment bias, hidden agenda bias, and variability caused by random error related to problems with unintentional data entry oversight and neglect.
Classification of Evidence and Guideline Recommendation Formulation
The concept of linking evidence to recommendations has been further formalized by the American Medical Association (AMA) and many specialty societies, including the American Association of Neurological Surgeons (AANS), the Congress of Neurological Surgeons (CNS), and the American Academy of Neurology (AAN). This formalization involves the designation of specific relationships between the strength of evidence and the strength of recommendations to avoid ambiguity. In the paradigm for therapeutic maneuvers, evidence is classified into that which is derived from the strongest clinical studies (eg, well-designed, randomized controlled trials), or class I evidence. Class I evidence is used to support recommendations of the strongest type, defined as level 1 recommendations, indicating a high degree of clinical certainty. Nonrandomized cohort studies, randomized controlled trials with design flaws, and case-control studies (comparative studies with less strength) are designated as class II evidence. These are used to support recommendations defined as level 2 reflecting a moderate degree of clinical certainty. Other sources of information, including observational studies such as case series and expert opinion, as well as randomized controlled trials with flaws so serious that the conclusions of the study are truly in doubt are considered class III evidence and support level 3 recommendations, reflecting unclear clinical certainty. A summary of these categories of evidence can be viewed at Joint Guideline Committee Guideline Development Methodology document.
Results
Microsurgical Approach And Presence Or Absence Of Serviceable Hearing
Question 1
What surgical approaches for VSs are best for complete resection and facial nerve (FN) preservation when serviceable hearing is present?
Target Population
These recommendations apply to adults with sporadic VSs who underwent microsurgical resection via the RS or MF approach.
Recommendation
There is insufficient evidence to support superiority of either the MF or RS approach for complete VS resection and FN preservation when serviceable hearing is present.
Study Selection and Characteristics
The initial search strategy included 2949 candidate articles. A total of 218 articles were removed because they were outside the date range specified by the inclusion/exclusion criteria. After title and abstract review, 205 articles remained for full-text review. From these, 17 articles were included in the final review for question 1. Eight articles remained after the inclusion/exclusion criteria were applied and are summarized in Table 1 below. 14–21 Data extraction included study design, class of evidence, total number of patients, study selection parameters, mean or median tumor size, mean or median follow-up, and exclusion of NF2.
Results of Individual Studies, Discussion of Study Limitations, and Risk of Bias
Two main microsurgical approaches were analyzed for hearing preservation (HP) and FN function preservation in VS patients when serviceable hearing was present at the time of surgery. Both the RS and MF approaches afford the opportunity to preserve hearing during VS resection. All of the studies analyzed were retrospective and had level 3 evidence. The House–Brackmann (HB) scale22 was used to classify FN function results. The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) criteria23 or the Gardner–Robertson (GR) hearing scale were used to classify hearing results. Both pure tone average (PTA) and speech discrimination (by percentage) were needed to evaluate hearing preservation. AAO-HNS class A or B hearing or GR hearing grade I to II was considered functional or serviceable hearing. HB grade I/II FN function was used as the standard for normal to good FN function after surgery. Hearing preservation was defined as a class A or B or grade I to II result. At least 12 months of clinical follow-up of patients was also required to be included in the final analysis.
Of the 17 articles examined, 9 articles did not include the AAO-HNS hearing criteria, GR hearing scale grading, or clinical follow-up for ≥12 months and were therefore excluded. In the remaining 8 articles, analysis of FN function and HN preservation was made with the RS and MF microsurgical approaches. Four articles examined the results of the RS and MF approach for VS resection. Two articles examined the results of the RS approach only, while 2 articles focused on the MF approach.
Successful HP and FN function were found in patients undergoing an MF microsurgical approach for resection of their VS.14–21 The MF approach is selected mainly for patients with intrameatal VS tumors. Functional HP rates of 18.9% to 77% were reported with the MF approach. FN function preservation rates (HB I) were between 50% and 86%.14–16,18,19,24
The RS approach also provided excellent HP and FN function after VS resection.14,17–21 HP rates between 11% and 68% were found in patients undergoing an RS approach. FN function preservation rates ranged between 59% and 98.7%.
There were 3 studies analyzing both the MF and RS approaches for VS resection that included data on HP and FN function.14,18,19 In those studies, HP was higher with the MF approach, while FN function preservation was greater with the RS approach.
Because all of the selected publications were either retrospective or nonrandomized prospective studies, there is a substantial risk of treatment selection bias. Specific to microsurgery for hearing preservation, ideal candidates with good existing hearing and small tumor size, are considered for hearing preservation. In addition, because most studies only included a single treatment arm, it is difficult to isolate the contribution of surgery to the immediate and delayed deterioration of hearing decline from the natural history of progressive decline inherent in having a VS. Finally, hearing preservation outcome analysis is particularly problematic for RS craniotomy, because the intent of hearing preservation is not always adequately outlined in the study. Specifically, some surgeons prefer the RS approach even in cases where hearing preservation is not attempted, such as for medium or large-sized tumors (>2 cm).
Tumor selection by approach also comes into play when comparing RS or MF craniotomy. Generally, only small lateral-based tumors are managed with an MF craniotomy. This is compared to the RS approach, where larger and more medial-based tumors are often approached. If tumor size is not adequately adjusted for, these inherent selection biases would favor improved outcomes for MF in comparison to the RS approach. Therefore, when comparing outcomes, it is critical that the same size class is compared because size is one of the primary predictors of hearing preservation and FN outcome. Finally, reporting bias must be considered. Specifically, series with better patient outcomes are more likely to be reported compared to series with mediocre or suboptimal surgical results.
Synthesis of Results/Discussion
Both the MF and RS surgical approaches can permit preservation of hearing and FN function. Small, lateral-based VS tumors in the IAC may permit greater hearing preservation by an MF approach. FN preservation rates are reported higher with an RS approach in patients with serviceable hearing undergoing surgical resection of their VS.
The evidence for this guideline was drawn from studies with class III evidence; currently, no class I or II evidence exists to guide recommendations for this subject. These data should be used when counseling patients regarding the probability of long-term maintenance of serviceable hearing and FN preservation after microsurgery for sporadic VSs.
Question 2
What surgical approaches for VSs (RS or translabyrinthine [TL]) are best for complete resection and FN preservation when serviceable hearing is not present?
Target Population
This recommendation applies to adults with sporadic VSs who underwent microsurgical resection via the RS or TL approach.
Recommendation
There is insufficient evidence to support superiority of either the RS or TL approach for complete VS resection and FN preservation when serviceable hearing is not present.
Study Selection and Characteristics
The initial search strategy included 2949 candidate articles. A total of 218 articles were removed because they were outside the date range specified by the inclusion/exclusion criteria. After title and abstract review, 205 articles remained for full text review. From these, 29 articles were included in the final review for question 2. Twenty-two articles remained after the inclusion/exclusion criteria were applied, and are included in Table 2 below.25–46 Data extraction included study design, class of evidence, total number of patients, study selection parameters, mean or median tumor size, mean or median follow-up, and exclusion of NF2.
Results of Individual Studies, Discussion of Study Limitations, and Risk of Bias
Two microsurgical approaches (RS and TL) were analyzed to determine the best approach for VS resection and FN function preservation in patients with nonserviceable hearing who had ≥12 months of clinical follow-up after their surgery. An MF approach is mainly used for patients with serviceable hearing and intracanalicular VS tumors and was not examined in this analysis. Most of the studies in this analysis classified normal to good FN function as HB grade I/II.
A total of 29 articles were reviewed; 7 studies were excluded because of incomplete clinical follow-up. The remaining 22 studies included 3 nonrandomized prospective studies25–27 and 19 retrospective studies.28–46 The HB scale22 was used for classification of FN function results.
A total of 16 studies described the RS approach and provided detailed FN functional preservation rates in patients who underwent VS resection.25, 27–34,37,40–45 Fifteen studies described the TL approach for VS resection and FN functional preservation.26,27,29,34–39,41–46 Eight studies compared the TL approach with the RS approach for VS patients undergoing surgery with nonserviceable hearing.29,34,37,41-45
Among patients undergoing an RS approach and complete VS resection, normal FN function (HB I) ranged from 36% to 95%, while good FN function (HB I/II) ranged from 67 to 95%. The size of the tumor was a confounding variable as the larger sized tumors had lower FN function preservation.
Among patients undergoing a TL approach, FN function preservation rates (HB I) ranged from 29% to 89%. When comparing FN function preservation rates in patients either undergoing an RS or TL approach for complete VS resection at the same center, some studies stated that a TL approach provided better FN function preservation.26,41 Other studies did not show a difference in FN function preservation.29,34,37,42,43
Because all the selected publications were either retrospective or nonrandomized prospective studies, there is a substantial risk of treatment selection bias. Tumor selection by approach also comes into play when comparing RS or TL approaches. Surgeon preference may be biased toward an RS approach because the TL approach usually requires the assistance of a neurotologist. In addition, larger VS tumors (>3 cm) have been typically resected by an RS approach instead of a TL approach because of the smaller bony opening with a TL approach. However, some groups prefer the TL approach for large VS tumors and contend that tumor size is not an obstacle when using extended or modified TL approaches.
Synthesis of Results/Discussion
Both the TL and RS approaches permit FN function preservation in patients with no serviceable hearing undergoing complete removal of VSs. The evidence for this guideline was drawn from studies with class III evidence; currently, no class I or II evidence exists to guide recommendations on this subject. These data should be used when counseling patients regarding the probability of FN preservation after microsurgery for sporadic VSs when nonserviceable hearing is present.
Question 3
Does VS size matter for facial and vestibulocochlear nerve preservation with surgical resection?
Target population
This recommendation applies to adults with sporadic VSs who underwent microsurgical resection via the TL, RS, or MF approach.
Recommendation
Level 3: Patients with larger VS tumor size should be counseled about the greater than average risk of loss of serviceable hearing.
Study Selection and Characteristics
The initial search strategy included 2949 candidate articles. A total of 218 articles were removed because they were outside the date range specified by the inclusion/exclusion criteria. After title and abstract review, 205 articles remained for full-text review. From these, question 3 had 44 final articles included in Table 3 below.14–16,24,25,31,34–38,41–43,47–76 Data extraction included study design, class of evidence, total number of patients, study selection parameters, mean or median tumor size, mean or median follow-up, and exclusion of NF2.
Results of Individual Studies, Discussion of Study Limitations, and Risk of Bias
The key results of individual studies are outlined in Table 3 below, and are summarized within the guideline recommendations. In total, there were 3 prospective, 1 cross-sectional, and 40 retrospective studies with proper clinical follow-up of ≥12 months. The results of a select group of prospective studies are summarized below.
In 2015, Chovanec et al25 reported the results of a prospective study analyzing 89 consecutive patients with unilateral VSs who underwent microsurgical resection via the RS approach. The primary objective of the study was to ascertain predictors of tinnitus after surgery; however, other factors, including hearing preservation, were also analyzed. They determined that the primary preoperative predictors of hearing preservation were tumor size/grade and preoperative hearing levels. Specifically, the mean tumor size of patients who had successful hearing preservation surgery was 19 mm (range 11–40 mm) compared to 29 mm (range 9–59 mm) for those who lost serviceable hearing (P < .01).
In 2003, Couloigner et al35 analyzed clinical and histologic parameters in a prospective cohort of 35 consecutive patients who underwent TL resection to determine associations with postoperative FN function. They found that tumor staging, absent or desynchonized homolateral ABR, audiovestibular signs of brainstem compression, histologic signs of inflammation, presence of tumor edema, and p53 protein–positive immunostaining were correlated with FN function. Most factors that predicted postoperative function, however, were correlated with tumor stage.
Data from the 38 retrospective studies largely corroborated these results. Collectively, these data demonstrate that tumor size is among the most reliable prognostic factors for hearing preservation and FN function after microsurgery of VSs.
Because all the selected publications were either retrospective or nonrandomized prospective studies, there is a substantial risk of treatment selection bias. Specific to microsurgery for hearing preservation, usually only ideal candidates, including those with good existing hearing and small tumor size, are considered for hearing preservation. In addition, because most studies only included a single treatment arm, it is difficult to isolate the contribution of surgery to the immediate and delayed deterioration of hearing decline from the natural history of progressive decline inherent in having a VS. Finally, hearing preservation outcome analysis is particularly problematic for RS craniotomy, because the intent of hearing preservation is not always adequately outlined in the study. Specifically, some surgeons prefer the RS approach even in cases where hearing preservation is not attempted, such as for medium or large-sized tumors.
Tumor selection by approach also comes into play when comparing RS, TL, or MF craniotomy. Generally, only small lateral-based tumors are managed with an MF craniotomy. This is compared to the RS approach, where larger and more medial-based tumors are often approached. If tumor size is not adequately adjusted for, these inherent selection biases would favor improved outcomes for MF vs RS craniotomy. Therefore, when comparing outcomes, it is critical that the same size class is compared because size is one of the primary predictors of hearing preservation and FN outcome. Finally, reporting bias must be considered. Specifically, series with better patient outcomes are more likely to be reported compared to series with mediocre or suboptimal surgical results.
Synthesis of Results/Discussion
Class III evidence supports the conclusion that tumor size is a strong predictor of hearing preservation and FN preservation after microsurgery resection.
The evidence for this guideline was drawn from studies with class III evidence. Currently, no class I or II evidence exists to guide recommendations on this subject. These data should be used when counseling patients regarding the probability of long-term maintenance of serviceable hearing and FN preservation after microsurgery for sporadic VSs.
Small Intracanalicular Vs Tumors And Surgical Resection
Question 4
Should small intracanalicular tumors (< 1.5 cm) be surgically resected?
Target Population
This recommendation applies to adults with sporadic VSs who underwent microsurgical resection.
Recommendation
There are insufficient data to support a firm recommendation that surgery be the primary treatment for this subclass of VS.
Study Selection and Characteristics
A total of 36 articles were identified by the search criteria, of which 13 were included for final analysis (Table 4).67,77–88 Other articles were excluded primarily because of the inclusion of tumors not solely confined to the IAC.
Results of Individual Studies, Discussion of Study Limitations, and Risk of Bias
While little controversy accompanies the management of large VSs that abut or compress the brainstem, the appropriateness of surgery for intracanalicular VSs continues to inspire debate. The natural history of this subset of tumors, when studied independently, appears to be that growth and some degree of hearing loss is expected over reported follow-up intervals. Pennings et al79 followed 47 patients with IAC VSs for a mean period of 3.6 years, noting growth rates and hearing status in this group of untreated patients. A total of 40% of patients experienced ≥2 mm of growth during this period. Seventy-four percent of patients retained useful hearing (classes A and B) during follow-up, and 65% of patients preserve hearing in the class A range. Interestingly, hearing loss was similar across patients with stable, growing, and shrinking tumors. Patients whose hearing did decline did so earlier in the follow-up period.
Other authors have reported growth rates of between 67.5% and 76.6%.80,84 Thomsen et al,84 following 40 patients with IAC VSs, noted a mean change of 3.2 mm over a follow-up period of 3.6 years, while Roche et al,80 in following a cohort of 47 patients with IAC VSs prospectively for a mean of 3.65 years, observed an average of 2.8 mm of growth in 76.6% of patients. More than 20% (20.3%) of patients remained stable. When hearing status was examined, 60% of patients retained their original hearing class, while 37.5% presented with a >10-dB hearing loss. Among patients with growing tumors, 32% lost useful hearing. Stangerup et al88 found that only 17% of prospectively followed intracanalicular tumors grew after mean follow-up of approximately 4 years and 70% of patients who had 100% speech discrimination score (SDS) at presentation still had class A hearing 10 years later.
Several groups have reported operative outcomes in IAC VSs. In a series of 26 patients with IAC tumors, as defined by a medial projection of <4 mm from the porus, operated on via the RS approach, facial and cochlear nerves were preserved in 100% of cases, with 96% of patients’ facial function graded HB I or II at follow-up.87 Serviceable hearing, defined here by SRT ≤50 dB and SDS ≥60%, was retained in 50% of patients. Samii et al’s series67 of 16 cases of IAC tumors showed similar results in which the RS approach was used. Facial and cochlear nerves were left intact, and resections were complete. One hundred percent of patients had normal facial function, and hearing was preserved in 57% of patients. In this series, hearing was defined as: good, with a loss of <30 dB; fair, with a loss of 30 to 59 dB; bad, with a loss of 60 to 89 dB; poor hearing, with a loss of ≥90 dB; and deafness. Three patients’ hearing actually improved postoperatively. Of note, speech discrimination scores were not given. Interestingly, vertigo and tinnitus were present in >75% of patients, with resolution of vertigo in all patients. Other authors note good results with a posterior fossa approach. Yamakami et al,77defining useful hearing as class A/B,89 wherein the PTA was ≤50 dB, and the speech discrimination score was ≥50%, included 6 solely IAC VSs in a broader analysis of the use of intraoperative ABR and CNAP during the resection of small VSs. Their resection rates were 100%, and the maintenance of useful hearing was 60%.
A number of groups have also reported surgical outcomes from the MF approach. Shelton and Hitselberger85 reported the House experience in 39 IAC VSs <0.5 cm, with excellent results. All tumors were completely resected, and HB I or II facial function was achieved in 97% of patients. Hearing was measured as follows: good (SRT ≤30 dB and SDS ≥70%), serviceable (SRT ≤50 dB and SDS ≥50%), and measurable (any measurable hearing). In their series, good postoperative hearing was preserved in 46.2% of patients; serviceable, in 59.4%; and measurable, in 67.5%. In parallel, Wigand et al86 reported their results with the extended middle cranial fossa approach, noting a 100% rate of resection in 25 cases of IAC VSs. The cochlear nerve was preserved in 100% of patients and, strikingly, 71% of patients had gross hearing preservation, with 48% of patients who had SRT ≤60 dB preoperatively maintaining this level after surgery. In a series of 103 patients who were largely IAC VSs operated on via the MF approach, Wang et al78 reported that 98% of tumors were completely resected, 91% of patients had HB I or II at 5 years, and the hearing preservation rates were high. In the early postoperative period, of the patients presenting with class A hearing, 67% remained class A, 17% were class B, 1% were class C, and 15% were class D. Of patients presenting with class B hearing, 24% were class A, 53% remained class B, 6% were class C, and 18% were class D. Of patients with class A hearing at follow-up, 65% retained that status at 5 years. 67% of patients with class B were class B at 5 years. Kumon et al82 reported on complete resections in 15 IAC VSs operated on by the MF approach; 66% of patients had at least serviceable hearing postoperatively (PTA >50 dB and SDS >50% as serviceable). However, only 75% of patients had grade I or II facial function at 1 year.
Other studies have reported results from both approaches. Haines and Levine83 advocated for early surgery given their results in resecting 12 IAC VSs for which hearing could be preserved through either the RS or MF approaches. Ten of 12 patients had HB I facial function, and their hearing preservation rate was 82%. The authors suggested that a demonstration of improved outcome with resection of small tumors should spur resection of smaller tumors. Colletti et al81 operated on 50 patients with IAC VSs (25 with the RS approach, and 25 through the MF approach). FN function was better in the early postoperative period in the RS group than in the MF group (80% grade 1 and 2 vs 56%). At 1 year, 92% of patients in the RS group had grade 1 and 2 facial function as compared with 80% in the MF group, although this difference was not statistically significant. Hearing preservation, defined by class A to C hearing, was similar in each group, with 57% in the RS group and 66% in the MF group meeting these criteria. The MF approach was superior when tumors were ≤3 mm from the IAC fundus. In this cohort, hearing preservation (class A–C) was achieved in 69.9% of patients in the MF group compared to 44% in the RS group. Smaller IAC tumors (<7 mm) were associated with improved rates of hearing preservation, as were tumors associated with minimal enlargement of the IAC.
Synthesis of Results/Discussion
Excellent rates of resection, FN preservation function results, and hearing preservation have been reported after surgery for IAC VSs. However, there are insufficient data to support a firm recommendation that surgery be the primary treatment for this subclass of VS. A comparison study between surgery, observation, and SRS for IAC VSs may provide better evidence to support one treatment over the other.
Routine Hearing Preservation and VS Surgical Resection
Question 5
Is hearing preservation routinely possible with VS surgical resection when serviceable hearing is present?
Target Population
This recommendation applies to adults with both sporadic and NF2 VSs undergoing microsurgical resection via the MF or RS approach.
Recommendation
Level 3: Hearing preservation surgery via the MF or the RS approach should be attempted in patients with small tumor size (< 1.5 cm) and good preoperative hearing.
Study Selection and Characteristics
The authors reviewed 2949 articles relating to hearing preservation after VS surgery. From this cohort, we identified 169 articles describing outcomes of hearing preservation after VS surgery. Most studies were excluded because of insufficient data or incomplete follow-up. The most common reason for exclusion was absent data for audiologic assessment. The remaining 27 articles are included for this analysis (Table 5).17,19,52,57,69,81,90–110
Synthesis of Results/Discussion
Information extracted included study design, level of evidence (class), number of patients with VSs (including overall starting cohort), hearing classification scale, tumor size and location, preoperative hearing level, surgeon’s experience, tumor adherence studies, surgical approach, variable of meatal and fundus filling, extrameatal tumors (medial tumors), nerve where tumor arose, neurophysiology monitoring, hearing stability (long-term hearing preservation), patient perceived disability in regards to hearing loss, and inclusion of NF2 patients.
Results of Individual Studies, Discussion of Study Limitations, and Risk of Bias
The 27 articles consisted of 5 prospective, nonrandomized studies and 22 retrospective studies. The individual studies are presented in Table 5. A few representative studies are highlighted below.
Nonaka et al,17 in 2013, reported the hearing outcomes in a consecutive series of 410 unilateral VSs operated via the RS approach. Hearing preservation was attempted in 170 patients with tumors <20 mm, and overall 75.9% of patients had postoperative useful hearing using the AAO-HNS and the Sanna–Fukushima hearing scale. In addition, in patients with preoperative hearing classified as AAO-HNS class A or B and with tumors <21 mm, 82.8% retained useful or serviceable hearing postoperatively. Lastly, the authors report hearing preservation rates depending on the resection accomplished: 63.6% in GTR, 83.3% in STR, and 100% in NTR.
Sameshima et al,19 in 2010, reported hearing preservation rates between the MF and RS approach for tumors <1.5 cm. Hearing preservation indicated AAO-HNS class B or better. The authors report 76.7% hearing preservation via the MF approach and 73.2% via the RS approach. Samii et al,105 in 2006, reported hearing preservation rates for 200 consecutive patients with VSs resected via the RS approach. Overall, functional hearing preservation of 51% was achieved by their hearing classification scale. Hearing was graded according to the New Hannover Classification. Hearing classes H1 to H3, corresponding to a PTA of ≤60 dB and a speech discrimination score of >40%, were defined as functional. This hearing preservation rate varied depending on tumor size: 57% for small tumors to 42% in larger tumors.
In 2004, Sanna et al106 reported on hearing preservation rates using either the enlarged MF versus the RS approach and using both the AAO-HNS and modified Sanna classifications for hearing rates. Their cohort of 793 tumors included 107 hearing preservations surgeries, including NF2 patients. Importantly, the authors described how using various classification systems to measure postoperative hearing can give a false sense of success. Specifically, when they applied the AAO-HNS classification their hearing preservation results consist of 62.7% and 54.2% in the MF and RS approaches, respectively. When using the same data for the modified Sanna scale, their results drop down to 32.2% and 31.3% for the MF and RS approaches, respectively.
The 1994 study by Brackmann et al92 reported hearing preservation results using the MF craniotomy in patients with VSs. They achieved 71% hearing that was as good, better, or almost as good as preoperative scores using the best PTA of air or bone at 4 different frequencies.
All the reviewed articles were either retrospective or nonrandomized prospective studies with inherent risk for bias. Particularly important, specific centers conform to a unique hearing classification scale not universally used, thus making interpretation of audiological data cumbersome. In addition, centers prefer a certain microsurgical technique regardless of tumor size and preoperative hearing status (the case for RS craniotomy). There exists controversy and bias regarding the identification of appropriate candidates for surgery, choosing the surgical approach, and defining successful results. Therefore, most centers try to express their results in a way that appears more successful (for example, the study that used the AAO-HNS versus the GR scale). Lastly, skull base centers tend to describe better hearing outcomes than suboptimal outcomes compared to the available published literature, which is an inherent reporting bias.
Synthesis of Results/Discussion
Class III evidence suggests hearing preservation surgery using both the MF or the RS approach for removal of small to medium VSs in patients with good preoperative hearing function. The definition of hearing success after VS resection remains controversial. Many audiologic classification schemes have been developed to determine “hearing preservation,” and the fact that there are multiple schemes indicates that none is universally accepted.
VS Surgical Resection as Primary Treatment in NF2 Patients
Question 6
When should surgical resection be the initial treatment in patients with NF2?
Target Population
This recommendation applies to patients meeting diagnostic criteria for NF2.
Recommendation
There is insufficient evidence that surgical resection should be the initial treatment in patients with NF2.
Study Selection and Characteristics
The authors reviewed 2949 articles on surgical resection of VSs involving NF2 patients. A total of 164 articles were identified for full-text review, and 6 articles were included after full-text review (Table 6).93,111–115 Most articles were excluded as they involved a mixture of both sporadic VSs and NF2 VS tumors, insufficient data, or incomplete follow-up.
Results of Individual Studies, Discussion of Study Limitations, and Risk of Bias
In patients with NF2, early surgical therapy is an option aimed at preserving the patient’s long-term quality of life as the goal of therapy. This can be meaningfully achieved with hearing preservation when possible, or prevention of side effects secondary to tumor progression and mass effect. However, it should be noted that in expert hands hearing loss or complications can occur with surgery. Observation or stereotactic radiosurgery appear to be viable alternatives when considering quality of life. Here the level of clinical evidence for early surgical intervention in patients with NF2 is assessed.
Most of the surgical series and studies available actively excluded patients with NF2. Performing a thorough literature evaluation yielded only 6 studies with clinical evidence available to aid in assessing early surgical intervention. One series of studies from the House Institute published overlapping epical results relative to the MF approach and hearing preservation in patients with VSs.93,113,114 In intervals from 1988 to 1999, 1992 to 2004, and 2000 to 2011, the House Group reported excellent useful hearing preservation (between 60% and 48%) with minimal morbidity (overall good functional 7th nerve preservation) for smaller tumors (mean of 1.1 cm).93,113,114 High gross total resection rates were reported (>96%). However, the recurrence rate on MRI was reported as 59%, although in this disease it is difficult to determine if these were local or separate tumors given the nature of NF2.93,113,114 The largest series was written by Samii et al,112 who reported on 120 tumors in 82 NF2 patients through various approaches compared to a spontaneous VS cohort.112 This study reports nearly 40% useful hearing preservation with excellent FN preservation rates for a cohort that includes 82 tumors >3 cm.112 Notably, in this group compared to sporadic VSs, the authors noted that NF2-related tumors grow faster, and that anatomical and functional nerve outcomes are lower. They advocate for early surgery to improve results in these patients.112 Glasscock et al’s series111 notes similar findings to Samii et al’s and comes to the same conclusions of reduced functional nerve preservation and advocates for earlier intervention. Contrary to these authors’ observations, Tysome et al115 reports a similar series of larger tumors with poorer functional outcome compared to the sporadic acoustic neuroma experience. The authors concluded that intervention should occur at the time of first documented growth on imaging with emphasis placed on hearing preservation.115
Synthesis of Results/Discussion
In considering these series, evidence for early intervention appears favorable. However, given the high rate of hearing loss at the time of surgery and potential for secondary tumor development, observation is also a viable option as well as stereotactic radiation. The available evidence does not dissuade one from early intervention, However, clearly in experienced hands, the results are less favorable than in sporadic VSs.
Key Issues for Future Investigation
Given that there are few patients at any given center with this disease, it appears that it will be difficult for any one institution to perform a randomized trial on outcomes for these patients. Therefore, multi-institutional studies, either prospective or registry-based, should be undertaken. Although most studies report short-term impact on hearing and FN preservation, there are no data regarding timing of surgical intervention and patient survival. This is a difficult concept to weigh for most clinicians in that a surgeon can remove these tumors in their entirety, thereby preserving the brainstem from compression; however, the patient’s quality of life likely will suffer in the process if they suffer deafness and additional neuropathies. Given this complexity, the most pressing need to understand for further research is the interest of the patient and his or her general preference so this may drive the treatment paradigms.
Multidisciplinary VS Resection and CN Preservation
Question 7
Does a multidisciplinary team, consisting of neurosurgery and neurotology, provide the best outcomes of complete resection and facial/vestibulocochlear nerve preservation for patients undergoing resection of VSs?
Target Population
This recommendation applies to adults with sporadic VSs who underwent microsurgical resection.
Recommendation
There is insufficient evidence to support stating that a multidisciplinary team, usually consisting of a neurosurgeon and a neurotologist, provides superior outcomes compared to either subspecialist working alone.
Study Selection and Characteristics
A total of 10 articles were identified by the search criteria, of which 4 were included for final analysis (Table 7).116–119 Other articles were excluded because of a lack of focus on the concept of joint surgery for VSs.
Results of Individual Studies, Discussion of Study Limitations, and Risk of Bias
Neurosurgeons, neurotologists, and radiation oncologists routinely manage patients with VSs, and treatment may be performed by a single surgeon or as a team. Surgeons who resects these tumors may also vary. Neurosurgeons may work alone. Neurotologists may work alone; or, as is occurring with increasing frequency, neurosurgeons may work with a neurotologist, a partnership best promulgated by the famous partnership of William House (neurotology) and Bill Hitselberger (neurosurgery). Whether or not this team approach leads to improved outcomes is unclear.
A recent survey assessed practice patterns in the United States/Canada.117 Of 706 respondents, the majority of respondents (85.7%) treat VSs as part of a team, with 75.8% adding that it should be standard of care. Neurosurgeons from the southern United States were more likely to operate alone, and those with higher volumes were more likely to work in a team with an ear, nose, and throat specialist.117 A similar survey was published in 2006 assessing compliance in the United Kingdom and Ireland with the Clinical Effectiveness Guidelines for the management of acoustic neuromas produced in 2002 by the British Association of Otorhinolaryngologists – Head and Neck Surgeons (BAO-HNS).120 These guidelines made a number of recommendations, among which was a stipulation that there should be teamwork between neurosurgeons and ear, nose, and throat surgeons with a specialist interest in neurotology. Of 56 neurosurgeons performing such surgery, 75% work with a neurotologist. Those who operated alone did so solely through a posterior fossa approach and treated <10 patients per year.118 However, while these results suggest that most neurosurgeons practicing today in the United States/Canada and United Kingdom/Ireland work with an ENT specialist for VS resection, the effect of this partnership on outcomes was not addressed. To the authors’ knowledge, no studies exist comparing results with and without a team approach. Few studies directly address outcomes in the context of a surgical team approach.
Tonn et al119 reviewed their series of 508 cases where each surgery was performed by a neurosurgeon and neurotologist. With 88.7% HB I to III facial function at 6 months and 38.9% serviceable hearing, the authors, in part, attribute their results to the concept of team surgery, wherein the extracanalicular portion was resected by a neurosurgeon and the IAC portion by a neurotologist. One group extolling the merits of a combined approach concluded that they needed about 60 cases to achieve superior FN outcomes and improved resection rates, but these results were not compared to a single-surgeon approach.116 The question pertinent to treatment was easily searchable and limited, so missed studies are possible but unlikely. Given the rarity of the disease, subjects are quite limited and may lead to some bias.
Synthesis of Results/Discussion
There is insufficient evidence to recommend that VSs be resected with a neurosurgeon and a neurotologist, although the majority of high-volume skull base centers in the United States, Canada, United Kingdom, and Ireland use such a model during VS surgery.
VS Subtotal Resection Followed by SRS
Question 8
Does a subtotal surgical resection of a VS followed by stereotactic radiosurgery (SRS) to the residual tumor provide comparable hearing and FN preservation to patients who undergo a complete surgical resection?
Target Population
This recommendation applies to adults with sporadic VSs who underwent microsurgical resection.
Recommendation
There is insufficient evidence to support subtotal resection followed by SRS provides comparable hearing and FN preservation to patients who undergo a complete surgical resection.
Study Selection and Characteristics
For this topic, 17 full-text articles were reviewed, and 4 were excluded (Table 8).30,121–132 Two studies were excluded because their data were already reported in a large review article, which was included. Three other excluded studies simply discussed long-term follow-up after subtotal resection without an evaluation of patients who underwent secondary radiosurgery. One excluded study was an evaluation of microsurgery compared to SRS, and the final excluded study was of a patient population who underwent primary SRS.
Results of Individual Studies, Discussion of Study Limitations and Risk of Bias
Of the 13 included articles reviewed, all were retrospective reviews of patients who underwent radiosurgery after receiving subtotal VS resection. All of the papers discussed tumor control rate, and each discussed variably FN function or hearing preservation. None of the articles offer direct comparison to a gross total resection group but cite outcomes from other papers in their discussion.
Brokinkel et al125 conducted a review of 6 similar retrospective studies, including a total of 159 patients. FN function was spared in 142 of 151 patients with initial HB grade 1 or 2. Hearing remained serviceable in 15 of 129 patients with preoperative serviceable hearing. Tumor growth control was reported in 149 of 159 patients, with 6 requiring repeated therapy.
Pollock et al122 provided 2 reports on SRS after subtotal resection. The first study included 76 patients with a mean follow-up of 43 months. Eleven of 47 patients with HB grade 1 to 3 had increased weakness. Tumor growth control was reported in 73 of 78 tumors with 6 patients undergoing further surgery, and 1 undergoing repeat radiation. The second study evaluated 55 patients with a residual or recurrent tumor, which was treated a median of 60 months after resection, with 47 of these patients demonstrating enlarging tumors.123 The reported tumor control rate was 94%, and 4 of 42 patients with normal to moderate FN function developed weakness.
Unger et al124 reports on 86 patients with a median follow-up of 75 months. Their reported tumor control rate was 96%, with 5 patients developing grade 3 to 4 FN weakness, and no change in those with preoperative serviceable hearing.124
All of these studies were retrospective and are therefore subject to the inherent bias associated with any retrospective analysis. None of the included studies had their own internal control of patients undergoing gross total resection, but instead included some comparison to the results of other studies or largely generalized averages of hearing preservation and local tumor control. Without randomization, there is certainly inherent differences in tumor histology and anatomy that would play a role in whether a patient received primary gross total resection or subtotal resection. It is unknown what effect these pretreatment variables would have on outcomes regardless of treatment approach. The number of included studies is small with 4 included studies (1 being a review of 6 smaller studies) for a total of only 386 patients.
Synthesis of Results/Discussion
When a VS is treated with subtotal resection followed by radiosurgery either primarily or because of tumor remnant growth, tumor control rates are consistently 93% to 96% with >90% of patients maintaining normal or near normal facial function. This tumor control rate is similar to that of series on gross total resection; however, the FN function preservation is consistently better than the wide range of facial function preservation reported (31.4–92.8%) for gross total resection.
Additional Analysis/Future Research
Future studies directly comparing gross total resection to subtotal resection plus radiosurgery with regard to outcomes for similar patients with similar tumors on a prospective basis in regard to cranial nerve function as well as long-term tumor control would provide the strongest data to address the stated question. In addition, it would be highly valuable for a “lowest acceptable” percentage of surgical resection to be determined that could still be followed by radiosurgery with comparable results to gross total resection.
VS Resection and Preoperative Balance Difficulties
Question 9
Does surgical resection of VSs treat preoperative balance problems more effectively than SRS?
Target Population
This recommendation applies to adults with sporadic VSs who underwent microsurgical resection or SRS treatment.
Recommendation
There is insufficient evidence to support either surgical resection or SRS for treatment of preoperative balance problems
Study Selection and Characteristics
The authors reviewed 96 articles and identified 16 studies that addressed some aspect of the question of whether radiosurgery or open surgery influenced outcomes with respect to balance (Table 9).39,103,133–146 Studies that did not specifically address balance/vestibular dysfunction with respect to treatment or that did not contain quantitative analyses were excluded. Case reports and case series containing <10 patients were also excluded.
Ten of these studies looked specifically at balance improvement after treatment with either GK or surgery,39,103, 135–139,141,142,145 and 6 articles compared the two side-by-side. Patients were clinically evaluated in most studies. Six studies used questionnaires,133,134,140,143,144,146 most commonly the Dizziness Handicap Index (DHI).
Results of Individual Studies, Discussion of Study Limitations, and Risk of Bias
Two studies showed that treatment modality did not seem to influence outcomes.140,144 Three studies comparing SRS to microsurgery all concluded that patients undergoing microsurgery had worse balance outcomes.133,134,143 One study found that microsurgery patients had fewer vestibular problems ≤5 years after treatment, but that there was no significant difference between therapy groups >5 years after treatment.146 Four of these 6 studies are retrospective cohort studies, and the other 2 studies are case series. Each study uses a variety of endpoints assessed at different time spans. Reported incidence of new balance problems after surgery103,138,142 and SRS136,141 were also widely variable. Because 10 of the 12 studies are retrospective, these results are subject to case selection bias, bias caused by a loss of data, and publication bias. With surgery, however, subjective balance seemed to improve consistently in patients who presented with impaired balance.39,139,138 Notably, vestibular dysfunction was not associated with decreased quality of life.143
Synthesis of Results/Discussion
Vestibular symptoms seem to worsen in a minority of patients treated with both methods. A single study to determine the factors associated with improved balance after treatment is worthy of further exploration. Presently, there are limited data to support using SRS or microsurgery with the goal of improving balance, and what data exist are fraught with the expected selection biases, especially related to tumor size. In general, smaller tumors are treated with SRS and larger tumors are surgically resected. Tumor size, as a result, can be perceived as a significant confounding variable. In addition, the existing literature suggests that vestibular dysfunction is likely to be related to tumor size and patient age, among other factors. This makes the exact relationship between treatment modality and balance problems difficult to infer.
VS Resection and Trigeminal Neuralgia
Question 10
Does surgical resection of VSs treat preoperative trigeminal neuralgia more effectively than SRS?
Target Population
This recommendation applies to adults with sporadic VSs who underwent microsurgical resection or SRS treatment.
Recommendation
Surgical resection of VSs may be used to better relieve symptoms of trigeminal neuralgia than SRS.
Study Selection and Characteristics
For this topic, 12 full-text articles were reviewed, and 7 were excluded. One excluded study did not separate results based on tumor type, and 6 studies included <5 cases of VSs and trigeminal neuralgia and for that reason were excluded (Table 10).147–154
Results of Individual Studies, Discussion of Study Limitations, and Risk of Bias
Three studies addressed surgical resection in patients with VSs and concurrent trigeminal neuralgia symptoms. The largest series, by Puca et al,153 evaluated fifth nerve dysfunction in 136 middle and posterior fossa tumors, of which 88 patients had VSs. Twenty-five patients with VSs also had trigeminal symptoms ipsilateral to the side of the tumor. Results were not quantified by tumor type, but of 9 patients with middle and posterior fossa tumors examined who reported typical trigeminal neuralgia symptoms, 8 (88.9%) experienced complete or partial relief of pain symptoms. Another series by Barker et al152 consisted of 26 patients with posterior fossa tumors, all of whom reported typical trigeminal neuralgia symptoms, of which 8 patients had VS. Seven of 8 (87.5%) patients with VSs and trigeminal neuralgia experienced partial or complete relief of pain. Interestingly, in 23 of 26 cases of posterior fossa tumors examined, vascular compression of the ipsilateral fifth nerve was also noted at the time of surgery. Samii et al154 evaluated 9 patients with small VSs, not involving the brainstem, and all reporting concurrent typical trigeminal neuralgia symptoms. In all 9 patients, coexisting vascular compression of the ipsilateral fifth nerve was identified at the time of surgery. Nine of 9 patients experienced complete pain relief immediately after surgery and had continued complete pain relief at 6 months of follow-up.
Two studies addressed radiosurgical treatment of patients with VSs and concurrent trigeminal neuralgia symptoms. The largest series by Badakhshi et al147 consisted of 61 patients with VSs and trigeminal symptoms, of which 34 patients had trigeminal pain, and were treated with SRS or fractionated stereotactic radiotherapy. Ten of 61 patients (16.3%) had relief of trigeminal symptoms postoperatively, although the type and degree of relief was not defined. Interestingly, 18 of 189 (9.5%) patients experienced new trigeminal “dysfunction” after treatment. In a study by Squire et al,149 5 patients were treated with SRS at a median marginal dose of 12 Gy, and all but 2 prescriptions were to the 50% isodose line. The remaining patients were treated to the 45% and 52% isodose lines, respectively. Four of 5 (80%) patients had a treatment response (defined as BNI score of I–III). All studies are limited by the fact that they are retrospective analyses. In addition, the distinction of trigeminal symptoms versus true trigeminal neuralgia was not made in several studies.
Synthesis of Results/Discussion
Of the 12 full text articles reviewed, 8 articles sufficiently address the resolution of trigeminal symptoms after surgery and other modalities. Four studies of VSs treated with surgical resection had excellent results with >87.5% of patients reporting, at minimum, partial relief of trigeminal pain. Interestingly, Barker et al152 noted ipsilateral vascular compression in 23 of 26 cases, and Samii et al154 noted vascular compression in 9 of 9 cases. Four studies that focused on radiosurgical techniques also showed improvement in trigeminal symptoms after treatment, although not to the degree reported in the surgical series. The largest series by Badakhshi et al147 showed improvement in 16.3% of cases as well as development of new trigeminal symptoms (pain, numbness, and hypesthesia) in 9.5% of patients. The 3 other studies showed better relief of symptoms (rates of 66%, 75%, and 80% by Koh et al,150 Prasad et al,151 and Squire et al,149 respectively).149-151 Unfortunately, the definition for improvement in trigeminal symptoms was not consistent across all studies, and some studies included trigeminal symptoms and not just true trigeminal pain symptoms.
Trigeminal neuralgia is rarely found in conjunction with ipsilateral VSs. This infrequent coincidence likely explains the relatively small and retrospective nature of the 8 relevant studies examined here.
Both radiosurgery and surgical resection can provide relief of trigeminal neuralgia in patients with VSs. Interestingly, 2 reports found high degrees (88% and 100%) of direct vascular compression of the trigeminal nerve at the time of surgery for VS. Surgery can permit microvascular decompression of the trigeminal nerve that can provide relief of trigeminal neuralgia. Given the well-established association between facial pain symptoms and vascular compression of the trigeminal nerve in “idiopathic” trigeminal neuralgia, these observations provide interesting although preliminary support of the hypothesis that vascular compression plays a role in pathophysiological mechanisms producing trigeminal neuralgia in some patients with VSs.
Additional Analysis/Future Research
Future studies that specifically correlate the presence or absence of vascular compression on preoperative imaging (particularly with improved anatomic imaging sequences such as FIESTA MRI) with intraoperative observation of direct vascular compression would help ascertain the true etiology of trigeminal neuralgia in patients with VS.
VS Surgical Resection After Initial SRS Treatment
Question 11
Is surgical resection of VS more difficult (associated with higher facial neuropathies and subtotal resection rates) after initial treatment with SRS?
Target Population
This recommendation applies to adults with sporadic VS who underwent microsurgical resection after SRS treatment.
Recommendation
Level 3: If microsurgical resection is necessary after SRS, it is recommended that patients be counseled that there is an increased likelihood of a subtotal resection and decreased FN function.
Study Selection and Characteristics
Twenty-two full-text articles were reviewed for this topic, and 12 were excluded. Eight articles were anecdotal only without outcomes data, 2 lacked discussion of surgical outcomes/details, 1 was a letter to the editor not an original article, and 1 was in French (Table 11).155–164
Results of Individual Studies, Discussion of Study Limitations, and Risk of Bias
Surgery can become necessary after SRS treatment of VS most likely owing to tumor regrowth or recurrence. The key results of individual studies that analyze when microsurgery was performed for VS after SRS are outlined in Table 11 and are summarized within the guideline recommendations. In 2014, Lee et al155 expanded upon their previous study159 in 2010 to include an additional 6 cases for a total of 13 cases treated with microsurgical resection after SRS. Of these 13 cases, 12 patients had normal FN function at median follow-up of 71 months, although subtotal resection was performed. One patient had a malignant peripheral nerve sheath tumor, which resulted in poor FN function.
In 2014, Hong et al156 evaluated 15 patients who had surgery after previous surgery compared to 5 patients who had surgery after previous radiation. Patients with previous radiation had preserved or improved FN function in 4 of 5 patients at 28 months, and 3 of 5 patients had gross total resection.
In 2012, Gerganov et al157 analyzed 15 patients with previous radiation then surgery, 13 patients with previous surgery and radiation then surgery, and 30 patients without previous treatment. Patients without previous treatment had better anatomic FN preservation than in patients with prior radiation (93.3% vs 86.7%). FN outcomes (HB grade 1–2) were improved in patients without previous radiation (70% vs 57%). Patients with previous radiation and previous surgery had overall worse outcomes.
In 2011, Friedman et al158 evaluated 73 patients who underwent resection after various types of radiation. Gross total resection was achieved in 79.5% of patients, although facial function was better preserved in patients with subtotal resection. Of patients with HB grade 1 to 2 preoperatively, 65% maintained HB grade 1 to 2 postoperatively.
In 2009, Liscak et al160 reported on 5 patients undergoing surgery after SRS (3 of which had previous microsurgery). Patients had preoperative HB grade 1 to 3. All of the patients had gross total resection, and all patients had poor FN outcome with 4 patients having HB grade 6 and 1 patient with HB grade 4.
In 2008, Shuto et al161 reported on 12 patients operated on after SRS. All 12 patients had subtotal resection. Of 8 patients starting out with HB grade 1, 5 patients were HB grade 1 postoperatively, and 3 patients were HB grade 3 to 4. In addition, the authors felt “complete dissection of the FN and tumor was difficult in most operations because of severe adhesions or color change.”
In 2006, Pollock et al162 reported on 5 patients who had surgical resection after SRS. Two patients had gross total resection, which resulted in complete facial palsies. Three patients had near total resection with preserved FN function.
In 2005, Friedman et al163 reported on 38 patients with previous radiation followed by surgical resection and a cohort of size-matched nonirradiated tumors treated with surgical resection. The authors found that radiated tumors were more adherent to FN (89% vs 63%). They reported a lower gross total resection in the irradiated group (78.9% vs 97.4%). The authors also found that good facial function (HB grade 1–2) was less likely to be achieved in the irradiated group (37% vs 70%).
In 1998, Pollock et al164 reported on 7 patients who underwent microsurgery after radiosurgery and 6 patients who underwent previous microsurgery and radiosurgery before surgical resection. Gross total and near total resection were achieved in 7 of 13 and 4 of 13 patients, respectively. Anatomic preservation of FN was achieved in 10 of 13 patients. Preoperatively, 11 of 13 patients were HB grade 1. Postoperatively, 3 patients were HB grade 1 to 2 and 7 patients were HB grade 6. The operating surgeons stated, “in comparison with their experience in VS patients who had not undergone radiosurgery, the tumor was more difficult to resect in 8 patients, no different in 4 patients, and easier in 1 patient.”
All studies that were included in this analysis were retrospective in nature and therefore have biases inherent in that study method. In particular, many studies included anecdotal or relative evaluations of the extent of tumor adherence and difficulty of surgery.
Synthesis of Results/Discussion
Class III evidence supports STR in patients with previous radiation to preserve FN function. The evidence for this guideline was drawn from studies with class III evidence. Currently there are no class I or II evidence to guide recommendations on this topic. There were multiple studies with anecdotal reports on the experience of surgical resection after radiation, although there was no consensus that surgery was more difficult after radiation. The class III evidence that was available suggests that subtotal resection should be considered to preserve FN function if surgery is considered necessary after previous radiation therapy.
Conflict of Interest (COI)
The Vestibular Schwannoma Guidelines Task Force members were required to report all possible COIs prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of Task Force members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methods manuscript (here).
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons and the Tumor Section of the Congress of Neurological Surgeons and the American Association of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document.
Acknowledgments
The authors acknowledge the Congress of Neurological Surgeons Guidelines Committee for its contributions throughout the development of the guideline and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Guidelines Committee for its review, comments, and suggestions throughout peer review, as well as Dr. Jeffrey J. Olson and Mary Bodach, MLIS, for assistance with the literature searches. Throughout the review process, the reviewers and authors were blinded from one another. At this time, the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Sepideh Amin-Hanjani, MD, D. Ryan Ormond, MD, Andrew P. Carlson, MD, Kimon Bekelis, MD, Stacey Quintero Wolfe, MD, Chad W. Washington, MD, Cheerag Dipakkumar Upadhyaya, MD, and Mateo Ziu, MD.
Figures
Figure 1. PRISMA flow chart diagram
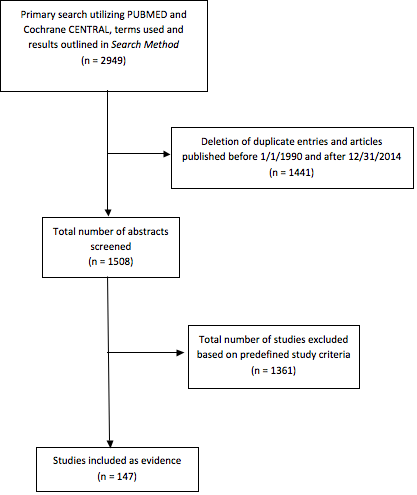
Table 1. Evidence table for question 1
|
Author, Year
|
Results
|
Data Class
|
Conclusions
|
|
Hillman et al, 2010
|
A retrospective case series, single institutional experience in 138 patients. Comparison of HP and FN function following RS and MF approaches for VS resection. All patients with FN weakness had ≥1 year of follow-up. Mean/median tumor size was 8 mm for MF and 14 mm for RS.
Among patients who underwent a MF VS resection, the following FN and hearing preservation outcomes were reported:
HB 1: 72%
HP success (class A or B): 59.3%
Among patients who underwent a RS resection of VSs, the following FN and hearing preservation outcomes were reported:
HB 1: 88%
HP success (class A or B): 38.5%
|
III
|
When classifying HB 1 and 2 together, there was no difference between MF and RS in regard to FN outcome. However, FN function recovered faster and there were more HB 1 in RS group.
There were more recurrent/residual tumors in the RS group and better hearing preservation in the MF group.
|
|
Meyer et al, 2006
|
A retrospective case series, single institutional experience, of 162 patients who underwent a MF approach for VS resection and attempted hearing preservation. Median follow-up was ≥12 months. Tumor size was (range 0.2–2.5 cm):
0.2–1.0 cm: 57%
1.1–1.4 cm: 21%
≥1.5 cm: 22%
|
III
|
Among patients who underwent MF resection of VSs, the following FN and hearing preservation outcomes were reported:
HB 1: 86%
HB 2: 10%
HB 3: 3%
HB 4: —
HB 5: —
HB 6: —
HP success (class A or B) was 57.3%
|
|
Kanzaki et al, 1997
|
A retrospective case series, single institutional experience, of 28 patients evaluating MF approaches for VS resection. Mean or median follow-up was 4.8 years (range 3–10 years).
Tumor size was:
Intracanalicular: 32%
<1 cm: 46%
>1 cm: 21%
|
III
|
Among 41 patients who underwent MF resection of VSs, the following FN and hearing preservation outcomes were reported:
HB 1: 68%
HB 2: 14%
HB 3: 18%
HP success (class A or B) was 36.7%
|
|
Nonaka et al, 2013
|
A large retrospective case series, single institution, evaluating 410 patients who underwent either a RS (71%), TL, or MF approach for VS resection. Hearing preservation attempted in RS patients with tumors that are small to medium. TL for large tumors and hearing loss. Median follow-up was 32.7 months. Tumor size was <2 cm in 204 patients (50%).
|
III
|
HN preservation was attempted in 170 patients (41.5%) with tumors <2 cm with good HN preservation in 74%.
FN preservation was HB 1 and 2 in 86% of patients. HB III–VI in 14% of patients (56 patients).
|
|
Rabelo de Freitas et al, 2012
|
A retrospective case series, single institution, evaluating 176 patients to compare HN and FN preservation with either MF (90 patients) or RS (86 patients) approach for VS. Median follow-up was 12 months.
Mean/median tumor size was 0.9 mm for MF tumors and 8 mm for RS tumors.
|
III
|
With MF, 80.7% had a HB I/II outcome. While patients who underwent a RS approach 96.5% had a HB I/II outcome. Better FN function after RS approach. No difference in outcomes with intracanalicular tumors.
There was no difference in hearing outcomes by Sanna classification system (classes A and B with 18.9% MF vs 10.6% RS).
|
|
Sameshima et al, 2010
|
A retrospective case series, single institution, evaluating 125 patients for HN preservation with small VS tumors. MF approach selected for tumor purely in the IAC while RS for tumors extending out porus.
Forty-three (43) patients underwent a MF approach while 82 patients underwent a RS approach. Follow-up was up to 12 months. Mean/median tumor size was 8.9 mm in the MF group while 12.4 mm in RS group.
|
III
|
Hearing was preserved in 76.7% of patients with MF approach vs 73.2% in the RS group. Temporary FN weakness was found more frequently in MF group. HB I found in 60.5% in MF while 98.7% in RS group early. RS approach provided several advantages over MF approach. MF required retraction, resulting in edema, speech difficulties, seizure, and longer operating room time.
|
|
Samii et al, 1997
|
Retrospective, single institution series evaluating 1000 VSs resected by RS approach evaluating FN function and hearing preservation. 979 tumors resected completely. 21 patients had deliberate partial removal in ill patients or those preserving hearing in good ear. Median follow-up was 12 months for 962 patients. Large tumors were >30 × 20 mm and small tumors were ≤30 × 20 mm.
|
III
|
FN preservation in 93%. 51% experienced normal FN function postop; 45% with reduced FN function; 59% with HB 1 and 2; HB 2 in 120 patients (13%); 140 patients (15%) HB 3; 60 patients (6%) HB 4; 100 patients (11%) HB 5; HN preservation in 68%.102 patients deaf in affected ear. Of 732 hearing ears preoperatively, 580 cochlear nerves were preserved in function with 39 patients with good, 115 patients with fair, and 135 patients with bad hearing. Hearing discrimination useful in 79% postoperative hearing patients.
|
|
Yang et al, 2008
|
Retrospective single institution series in 110 patients analyzing RS approach, and FN and HN preservation. The authors investigated prognostic factors for hearing preservation after removal of small VSs by the RS approach. The median follow-up was 23 months. Mean/median tumor size <2 cm.
Preservation of HB I/II function in 91% of patients; HN preservation and serviceable hearing (SDS ≥50 and PTA <50 dB) in 36%; all patients with useful preoperative hearing.
|
III
|
HN preservation influenced by tumor size and preoperative hearing; FN preservation of HB I/II in 81% postoperatively and 91% at 1–2 years; tumor size did not influence FN preservation in the small tumors; HN preservation and serviceable hearing (SDS ≥50 and PTA <50 dB) in 36%; all patients with useful preoperative hearing (AAO-HNS) class A or B.
|
AAO-HNS, American Academy of Otolaryngology-Head and Neck Surgery; FN, facial nerve; HB, House–Brackmann; HP, hearing preservation; IAC, internal auditory canal; MF, middle fossa; PTA, pure tone average; RS, retrosigmoid; SDS, speech discrimination score; TL, translabyrinthine; VS, vestibular schwannoma.
Table 2. Evidence table for question 2
|
Author, Year
|
Results
|
Data Class
|
Conclusions
|
|
Chovanec et al, 2015
|
Prospective cohort study, single institutional experience including 89 patients who underwent RS resection. Primary objective to evaluate tinnitus change. Follow-up range was 29–64 months and mean/median tumor size was 2.7 cm.
|
III
|
Among 89 patients who underwent RS resection of VSs, the following FN outcomes were reported:
HB 1–2: 67%
|
|
Dunn et al, 2014
|
Retrospective case series, single institutional experience, of 52 patients that underwent a VS resection by a RS approach. Median follow-up was 23 months. Mean/median tumor size was 3.5 cm (1.2–5.3)
|
III
|
Among 52 patients who underwent RS resection of VSs, the following FN outcomes were reported:
HB 1: 69%
HB 2: 8%
HB 3: 13%
HB 4: 2%
HB 5: —
HB 6: 4%
|
|
Moffat et al, 2014
|
Retrospective case series, single institutional experience, including 652 cases treated with RS or TL approach. FN function data obtained 2 years following surgery. The tumor size range was:
<1.5 cm: 19%
1.5–2.4: 36%
2.5–3.4: 23%
3.5–4.4: 14%
>4.5: 8%
|
III
|
Among patients who underwent TL resection of VSs, the following FN outcomes were reported:
<1.5 HB1–2: 79%
1.5–2.4 HB 1–2: 68%
2.5–3.4 HB 1–2: 52%
3.5–4.4 HB 1–2: 45%
>4.4 HB 1–2: 43%
Among patients who underwent RS resection of VSs, the following FN outcomes were reported:
<1.5 HB 1–2: 84%
1.5-2.4 HB 1–2: 82%
2.5-3.4 HB 1–2: 73%
Surgical approach not SS different on multivariate analysis.
|
|
Haque et al, 2011
|
Retrospective case series, single institutional experience, including 151 patients who underwent surgery, with 55 GTR and 96 STR by the RS approach. Median follow-up was 6 years (0.4–11.1 years). Mean/median tumor size was 3.3 cm (1.8–6 cm)
|
III
|
Among patients who underwent RS resection of VSs, the following FN outcomes were reported as HB 1–2: 97%
|
|
Misra et al, 2009
|
Retrospective case series, single institutional experience, comparing 100 cases done around 1993 to 100 cases performed around 2008, comparing completeness of resection, FN function. Recorded group around 2008 only given significant differences.
Number of patients: 100
Mean or median follow-up: –48 months (mean not given)
Mean or median tumor size:
Small (<2 cm): 4%
Medium (2–3 cm): 14%
Large (>3 cm): 82%
|
III
|
Among patients who underwent RS resection of VSs, the following FN outcomes were reported:
HB 1–2: 73%
HB 3: 14%
HB 4–6: 13%
|
|
Bae et al, 2007
|
Retrospective case series, single institutional experience including 163 patients, all RS approach. Correlated size and FN course.
Number of patients: 163
Mean or median follow-up: 62 months
Mean or median tumor size: 3.7 cm
|
III
|
Among 163 patients who underwent RS resection of VSs, the following FN outcomes were reported:
HB 1–2: 72%
HB 3–4: 22%
HB 5–6: 7%
|
|
Mirzayan et al, 2007
|
Retrospective review of 20 patients under the age of 21 who underwent RS approach with tumor resection.
Number of patients: 20
Mean or median follow-up: 6.9 years (4–10 years)
Mean or median tumor size: 3.5 cm (1–6 cm)
|
III
|
Among 20 patients who underwent RS resection of VSs, the following FN outcomes were reported:
HB 1: 95%
|
|
Darrouzet et al, 2004
|
Retrospective case series, single institutional experience, with 400 patients who underwent several different surgical approaches.
Number of patients: 400
Mean or median follow-up: 70 months
Mean or median tumor size: no measurements given, only Koos size
|
III
|
Among patients who underwent TL resection of VSs, the following FN outcomes were reported:
HB 1: 34%
HB 2–6: 66%
Among patients who underwent RS resection of VSs, the following FN outcomes were reported:
HB 1: 36%
HB 2–6: 64%
TL and RS FN outcomes not statistically significantly different.
|
|
Couloigner et al, 2003
|
Prospective cohort study, single institutional experience, evaluating 35 consecutive patients undergoing TL resection of VSs.
Number of patients: 35
Mean or median follow-up: “at least 1 year”
Mean or median tumor size: 2.0 cm (0–4.2 cm)
|
III
|
Among 35 patients who underwent TL resection of VSs, the following FN outcomes were reported:
HB 1: 49%
HB 2: 20%
HB 3: 11%
HB 4: 11%
HB 5: 6%
HB 6: 3%
|
|
Mamikoglu et al, 2002
|
Retrospective case series, single institutional experience, including 81 patients who underwent TL for large (>3 cm) VSs. FN outcomes are reported.
Number of patients: 81
Mean or median follow-up: 3.2 years
Mean or median tumor size: 3.7 cm (max 6)
|
III
|
Among 81 patients who underwent TL resection of VSs, the following FN outcomes were reported:
HB 1: 34%
HB 2: 11%
HB 3: 26%
HB 4: 8%
HB 5: 1%
HB 6: 19%
|
|
Guerin et al, 1999
|
Retrospective case series, single institutional experience, including 611 patients operated via RS, TL, and MF. FN outcomes by size and approach reported.
Number of patients: 611
Mean or median follow-up: 12 months FN function
Mean or median tumor size:
Small (<2.5): 61%
Moderate (2.5–4): 24%
Large (>4 cm): 15%
|
III
|
Among patients who underwent TL resection of VSs, the following FN outcomes were reported:
Not detailed, but 2.5% had HB 5–6
Among patients who underwent RS resection of VSs, the following FN outcomes were reported:
Not detailed, but 1.8% had HB 5–6
RS and TL were not statistically significantly different
|
|
Lanman et al, 1999
|
Retrospective case series, single institutional experience, including 190 large tumors (>3 cm) that underwent TL approach.
Number of patients: 190 patients
Mean or median follow-up: 12.6 months
Mean or median tumor size: 3.6 cm (3–7 cm)
|
III
|
Among 190 patients who underwent TL resection of VSs, the following FN outcomes were reported:
HB 1: 33%
HB 2: 20%
HB 3: 14%
HB 4: 15%
HB 5: 6%
HB 6: 13%
|
|
Andersson et al, 1997
|
Retrospective case series, single institutional experience including patients that underwent TL VS resection
Number of patients: 144
Mean or median follow-up: 3.3 years
Mean or median tumor size: 2.7 cm
|
III
|
Among 144 patients who underwent TL resection of VSs, the following FN outcomes were reported:
HB 1: 55%
HB 2: 16%
HB 3: 14%
|
|
Colletti et al, 1997
|
Retrospective case series, single institutional experience including 88 patients who underwent RS resection with either en bloc or debulking of VSs. Objective was comparison between these 2 strategies.
Number of patients: 88
Mean or median follow-up: 1-year postoperative HB scores provided
Mean or median tumor size: mean size for extracanalicular tumors was 1.6 cm
|
III
|
Among 88 patients who underwent RS resection of VSs, the following FN outcomes were reported:
HB I: 48%
HB 2: 32%
HB 3: 15%
HB 4: 4%
HB 5: 1%
HB 6: 1%
|
|
Grey et al, 1996
|
Retrospective case series, single institutional experience, including 276 patients who underwent TL and RS approach for VS resection. Comparison of 12-month postoperative function.
Number of patients: 276
Mean or median follow-up: 12-month results
Mean or median tumor size:
Categories:
Small 24%
Medium 32%
Large 44%
|
III
|
Among patients who underwent TL resection of VSs, the following FN outcomes were reported:
HB 1: 29%
HB 2: 19%
HB 3: 23%
HB 4: 8%
HB 5: 6%
HB 6: 15%
Among patients who underwent RS resection of VSs, the following FN outcomes were reported:
HB 1: 62%
HB 2: 17%
HB 3: 17%
HB 4: 2%
HB 5: -
HB 6: 2%
RS statistically significantly better than TL, but confounded by the fact that TL tumors were markedly larger.
|
|
Lalwani et al, 1994
|
Retrospective case series, single institutional experience, comparing RS, MF, and TL FN outcomes and also evaluating electroprognostic testing of FN.
Number of patients: 129
Mean or median follow-up: 1 year or greater for all
Mean or median tumor size:
<1 cm: 19%
1–3 cm: 62%
>3 cm: 19%
|
III
|
Among patients who underwent TL resection of VSs, the following FN outcomes were reported:
<1.0 cm HB1: 89%
1–3 cm HB1: 69%
>3 cm HB1: 64%
Among 45 patients who underwent RS resection of VSs, the following FN outcomes were reported:
<1.0 cm HB1: 86%
1–3 cm HB 1: 77%
>3 cm HB 1: 38%
No statistically significant difference between approaches.
|
|
Nissen et al, 1997
|
Retrospective case series, single institution, evaluating influence of 4 variables to FN outcome by review of 111 cases. Looked at tumor size, use of IOM, completeness of tumor resection, and surgical approach. TL approach in 47, 55 RS, and 9 combined cases.
>
Number of patients: 111
Mean or median follow-up: 12 months
Mean or median tumor size: no median, however most tumors <1.5 cm (51 patients).
|
III
|
FN function not found to be dependent on TL or RS approach.
Tumor size did not correlate FN functional outcome with no difference in significance. 90 patients with HB I or II function.
|
|
Sterkers et al, 1994
|
Retrospective series, single institution, analyzing preservation of AN function with different approaches. TL approach in 85% of patients. MF for intracanilicular tumors and RS approach for small tumors extending into the CPA. 34 cases operated on by RS approach and 22 cases by MF approach.
Number of patients: 573
Mean or median follow-up: 12 months
Mean or median tumor size: 37% (213) <2 cm, 50.5% (291) 2–4 cm, and 12.5% (72) 4 cm in diameter.
|
III
|
Hearing preserved in 8 cases of 22 MF cases (36.4%); hearing preserved in 13 cases (38.2%) of 34 patients by RS approach.
Overall result at 1 month was 77% of grade I or II function. For small tumors by TL route, 91–100% had grade I or II results, 76% achieved by means of MF approach, and 79% by RS approach. Results better with TL approach than with either the MF or RS routes.
Both at 1 month and 1 year, postop tumor size impacted FN function. Tumors <2 cm grade I/II was 70.9%, 2–4 cm was 36.9%, >4 cm was 20.8% at 1 month. Incidence of anatomic preservation and good facial function decreases with the size of the tumor.
|
|
Tos et al, 1998
|
Prospective single institution series that describes Danish experience with TL and MF approach for tumors; TL done in 703 tumors and MF in 19 tumors. Used suboccipital approach in 103 patients.
Number of patients: 103
Mean or median follow-up: 12 months
Mean or median tumor size:
>0–25 mm (305), 26–40 mm (197), >40 mm (190)
|
III
|
Total of 71% with HB I/II postoperative TL approach; 89% HB I/II for small (0–25), 71% for medium (26–40 mm), and 41% for >40 mm
TL approach better at FN preservation.
|
|
Van Abel et al, 2014
|
Retrospective single institution series comparing outcomes of patients ≥70 years of age to patients <70 years of age. Test a hypothesis that symptomatic tumors in older patients are more aggressive and higher rate of recurrence; matched cohort based on approach, completeness of resection, and tumor size; 4 patients had a GTR and 16 received a STR; RS in 11 patients and 9 underwent TL.
Number of patients: 20
Mean or median follow-up: 2.3 years
Mean or median tumor size: mean 3 cm
|
III
|
FN function worse in elderly patients
|
|
Zhang et al, 2012
|
Retrospective single institution series analyzing removal of large or giant VSs via TL approach. Divided into cystic (31%) vs solid (69%) groups of tumors. Total tumor removal was 89.6%.
Number of patients: 115
Mean or median follow-up: imaging follow-up at 1 year. Clinical follow-up postoperatively immediately.
Mean or median tumor size:
tumors >3.1 cm
|
III
|
29.6 % (30%) of patients had a HB I/II function at short term follow-up
|
|
Fenton et al, 2002
|
Single institution series assessing >predictive factors of long-term FN function; stratified by French and Australian nationalities; stress reporting FN function with postoperative Nl preoperative FN function and intact FN after surgery; middle fossa, TL, and RS approach; HB I in 55 patients; 45 TL, 8 RS, and 2 MF; no comparison of approaches.
Number of patients: 67
Mean or median follow-up: 24 months
Mean or median tumor size: median 2 and 2.4 cm
|
III
|
>Tumor size and minimum intensity to provoke a stimulus threshold factor for independent predictor of long term FN function; the bigger the tumor the greater the risk to the FN
|
AAO-HNS, American Academy of Otolaryngology-Head and Neck Surgery; CPA, cerebellopontine angle; FN, facial nerve; GTR, gross total resection; HB, House–Brackmann; HP, hearing preservation; IAC, internal auditory canal; IOM, intraoperative monitoring; MF, middle fossa; PTA, pure tone average; RS, retrosigmoid; SDS, speech discrimination score; STR, subtotal resection; TL, translabyrinthine; VS, vestibular schwannoma.
Table 3. Evidence table for question 3
|
Author, Year
|
Study Description
|
Data Class
|
Conclusions
|
|
Chovanec et al, 2015
|
Prospective cohort study, single institutional experience including 89 patients who underwent RS resection. Primary objective to evaluate tinnitus change.
Number of patients: 89
Mean or median follow-up: only range provided 29-64 months
Mean or median tumor size: 2.7 cm
|
III
|
Main prognostic factors of hearing preservation were size of tumor, preoperative hearing level, intraoperative neuromonitoring, tumor consistency, and adhesions to neurovascular structures.
|
|
Arnoldner et al, 2013
|
Prospective cohort study, single institutional experience. Prognostication of FN outcome based on “percent maximum” response. Primary outcome, 1-year HB score. All had TL approach only.
Number of patients: 78
Mean or median follow-up: 1.4 years
Mean or median tumor size: 2.3 cm
|
III
|
Age and sex did not have an impact on outcome, but tumor size did, with each cm increase in tumor size, patients were 105% more likely to have poor FN outcomes
|
|
Esquia-Medina et al, 2009
|
Prospective cohort study, single institutional experience, analyzing predictors of FN function (short and long-term) in 96 patients who underwent different approaches
Number of patients: 96
Mean or median follow-up: patients followed to 180 days
Mean or median tumor size:
Stage 1: 5%
Stage 2: 51%
Stage 3: 28%
Stage 4: 16%
|
III
|
Combination of tumor stage, adhesion, and nerve displacement in a logistic regression model was highly predictive of postoperative FN function.
|
|
Gerganov et al, 2009
|
Retrospective case series, single institutional experience, with 99 patients who underwent RS resection. FN outcomes evaluated at 2 weeks postoperatively.
Number of patients: 99
Mean or median follow-up: 2-week endpoint
Mean or median tumor size:
Hannover staging
T1: 9%
T2:10%
T3a: 19%
T3b: 15%
T4a: 33%
T4b: 13%
|
III
|
As the tumor size and volume increase, FN function is worse after surgery.
|
|
Hillman et al, 2010
|
Retrospective case series, single institutional experience. Comparison of HP and FN function following RS and MF.
Number of patients: 138
Mean or median follow-up: All patients with paralysis had at least 1 year of follow-up
Mean or median tumor size: 8 mm for MF and 14 mm for RS
|
III
|
Tumor size was analyzed by logistic regression and was not significantly related to FN function outcome.
|
|
Misra et al, 2009
|
Retrospective case series, single institutional experience, comparing 100 cases done around 1993 to 100 cases performed around 2008, comparing completeness of resection, FN function. Recorded group around 2008 only given significant differences.
Number of patients: 100
Mean or median follow-up: 1–48 months (mean not given)
Mean or median tumor size:
Small (<2 cm): 4%
Medium (2–3 cm): 14%
Large (>3 cm): 82%
|
III
|
Postoperative FN function was directly related to size of tumor (no statistical analysis to support).
|
|
Kim et al, 2006
|
Retrospective case series, single institutional experience, evaluating hearing preservation using MF and RS approach. Total of 93 patients evaluated. FN function not reported.
Number of patients: 93
Mean or median follow-up: NP
Mean or median tumor size: 1.3 cm
|
III
|
Variables of prognostic significance to hearing preservation included smaller tumor size, tumor location within the IAC, better preoperative hearing, and short latencies on ABR.
|
|
Meyer et al, 2006
|
Retrospective case series, single institutional experience, including 162 patients who underwent MF approach to tumor resection for attempted hearing preservation.
Number of patients: 162
Mean or median follow-up: ≥12 months
Mean or median tumor size: (0.2–2.5 cm):
0.2–1.0 cm: 57%
1.1–1.4 cm: 21%
≥1.5 cm: 22%
|
III
|
Tumor size and preoperative hearing status are important predictors of postoperative hearing results after MF surgery.
|
|
Baumann et al, 2005
|
Cross sectional study (mainly quality of life), single institutional experience, including 42 patients who underwent MF craniotomy for VS resection.
Number of patients: 42
Mean or median follow-up: 3.1 years
Mean or median tumor size: Intrameatal 49%
Intra and extrameatal 51%
|
III
|
Tumor location and size had no significant effect on changes of postsurgical facial paralysis
Tumor location and size had no significant effect on changes of postsurgical hearing ability
|
|
Darrouzet et al, 2004
|
Retrospective case series, single institutional experience, with 400 patients who underwent several different surgical approaches.
Number of patients: 400
Mean or median follow-up: 70 months
Mean or median tumor size: no measurements given, only Koos size
|
III
|
Poor FN outcome was correlated with tumor size and preoperative irradiation, not surgical approach.
|
|
Couloigner et al, 2003
|
Prospective cohort study, single institutional experience, evaluating 35 consecutive patients undergoing TL resection of VSs.
Number of patients: 35
Mean or median follow-up: “at least 1 year”
Mean or median tumor size: 2.0 cm (0-4.2 cm)
|
III
|
Factors predictive of postoperative FN function were tumor stage (size) and tumor edema.
|
|
Kobayashi et al, 2002
|
Retrospective case series, single institutional experience, including 45 patients undergoing MF approach for resection. Main objective was to analyze fundal cap and FN outcome.
Number of patients: 45
Mean or median follow-up: looked at 3-month FN outcomes following surgery
Mean or median tumor size: 1.1cm (0.4–2.0 cm)
|
III
|
Neither the length of the fundal CSF cap nor the tumor diameter had any correlation to the degree of postoperative FN palsy, immediately, or at 3 months after surgery.
|
|
Mamikoglu et al, 2002
|
Retrospective case series, single institutional experience, including 81 patients who underwent TL for large (>3 cm) VSs. FN outcomes are reported.
Number of patients: 81
Mean or median follow-up: 3.2 years
Mean or median tumor size: 3.7 cm (max 6)
|
III
|
Spearman rank test showed a correlation between FN function and tumor size.
|
|
Matthies et al, 2002
|
Retrospective case series, single institutional experience, including 1800 VSs removed by RS approach. Difficult to follow FN outcome numbers as presented.
Number of patients: 1800
Mean or median follow-up: not reported
Mean or median tumor size:
T1: 7%
T2: 15%
T3: 42%
T4: 36%
|
III
|
Hearing preservation was greatest in cases of small intrameatal or slightly extrameatal tumors. There is a marked decrease in hearing preservation for T3- or T4-sized tumors.
|
|
Ferber-Viart et al, 2000
|
Prospective cohort study, single institutional experience, including 107 hearing preservation attempts. Compares population with HP to non-HP group. Found that audiometric features were most predictive. FN data not presented.
Number of patients: 107
Mean or median follow-up: NP
Mean or median tumor size: overall number not published (only separate groups)
|
III
|
The size of the tumor and the preoperative hearing levels are long-standing predictive factors of hearing preservation for VSs.
|
|
Hahn et al, 2000
|
Retrospective case series, single institutional experience. Comparison of audiovestibular pre- and postoperatively for patients who underwent TL, RS, and MF resection of VSs. FN outcomes not reported.
Number of patients: 131
Mean or median follow-up: NP
Mean or median tumor size:
Small (<2.5): 39%
Medium (2.5–3.5): 47%
Large (>3.5): 6%
|
III
|
The preservation ratio of the cochlear nerve showed a negative correlation to tumor size. A trend toward higher success rates was seen with intracanalicular tumors.
|
|
Guerin et al, 1999
|
Retrospective case series, single institutional experience, including 611 patients operated via RS, TL, and MF. FN outcomes by size and approach reported.
Number of patients: 611
Mean or median follow-up: 12- month FN function
Mean or median tumor size:
Small (<2.5): 61%
Moderate (2.5–4): 24%
Large (>4 cm): 15%
|
III
|
Larger tumors had a higher incidence of poor FN function compared to smaller tumors.
|
|
Lanman et al, 1999
|
Retrospective case series, single institutional experience, including 190 large tumors (>3 cm) that underwent TL approach.
Number of patients: 190 patients
Mean or median follow-up: 12.6 months
Mean or median tumor size: 3.6 cm (3–7 cm)
|
III
|
FN outcome was related to tumor size, with poor results in 50% of patients with tumors >4 cm compared to 28% of those with tumors measuring 4 cm, and <10% of those with tumors <4 cm.
|
|
Kanzaki et al, 1997
|
Retrospective case series, single institutional experience, evaluating all MF and extended MF approaches for VS resection.
Number of patients: 28
Mean or median follow-up: 4.8 years (3–10 years)
Mean or median tumor size:
Intracanalicular: 32%
angle <1 cm: 46%
angle >1: 21%
|
III
|
Hearing preservation rate did not depend on the ABR pattern, tumor size, or origin of the tumor.
|
|
Colletti et al, 1996
|
Retrospective case series, single institutional experience 38 patients that underwent HP surgery. Objective was to identify risk factors for hearing loss.
Number of patients: 38
Mean or median follow-up: NP
Mean or median tumor size: 1.6 cm (0.5–2.4)
|
III
|
Increasing tumor size was associated with greater risk of damage to the cochlear nerve and hearing loss during hearing preservation surgery for VSs.
|
|
Grey et al, 1996
|
Retrospective case series, single institutional experience, including 276 patients who underwent TL and RS approach for VS resection. Comparison of 12-month postoperative function.
Number of patients: 276
Mean or median follow-up: 12- month results
Mean or median tumor size:
Categories:
Small 24%
Medium 32%
Large 44%
|
III
|
Increasing age and increasing tumor size were associated with worse FN outcome.
|
|
Leonetti et al, 1995
|
Retrospective case series, single institutional experience, including 168 cases that underwent surgery using the MF, RS, and TL approaches. FN outcomes were not separated by approaches.
Number of patients: 168
Mean or median follow-up: Not specified but most had minimum of 1 year
Mean or median tumor size:
0–1.0: 18%
1–2.0: 48%
2.0: 24%
>4.0: 10%
|
III
|
The incidence of FN paralysis is closely related to the size of the tumor resected. Similarly, the ability to save hearing was closely related to tumor size.
|
|
Kanzaki et al, 1994
|
Retrospective case series, single institutional experience, evaluating MF and extended MF for VS resection.
Number of patients: 69
Mean or median follow-up: NP
Mean or median tumor size:
Only reports “tumor size was 2 cm or less in 90% of cases and 2.1 cm or larger in 10%.”
|
III
|
The hearing preservation rate decreased according to the extension of the tumor into the posterior fossa. However, the hearing preservation rate was not significantly higher in the cases with a tumor of ≤3 mm than in those with larger tumors.
|
|
Lalwani et al, 1994
|
Retrospective case series, single institutional experience, comparing RS, MF, and TL FN outcomes and also evaluating electroprognostic testing of FN.
Number of patients: 129
Mean or median follow-up: 1 year or greater for all
Mean or median tumor size:
<1 cm: 19%
1–3 cm: 62%
>3 cm: 19%
|
III
|
Long-term FN function was inversely correlated with size of tumor, and was not related to side of tumor, age, sex, or surgical approach.
|
|
Arriaga et al, 1993
|
Retrospective case series, single institutional experience. Analysis of tumor volume and linear dimension correlation with preoperative hearing, and tumor volume and linear dimension and postoperative FN function.
Number of patients: 1036
Mean or median follow-up: NP
Mean or median tumor size: NP
|
III
|
Even small changes in tumor diameter (especially in larger tumors) can result in tumor volume changes that may be associated with significant changes in postoperative FN function.
|
|
Berges et al, 1993
|
Retrospective case series, single institutional experience including 43 patients who underwent TL resection of VSs. Goal of paper to evaluate electroprognostic of proximity to distal minimum stimulation ratio.
Number of patients: 43
Mean or median follow-up: all had at least 0.5 years (180 days)
Mean or median tumor size: cm sizes not given
|
III
|
Appears that size influences FN preservation based on numbers, but authors did not run statistics on this aspect.
|
|
Kirkpatrick et al, 1993
|
Retrospective case series, single institutional experience, evaluating 38 patients who received TL or RS approach for resection of VSs. FN outcomes by approach are not provided.
Number of patients: 38
Mean or median follow-up: 18.5 months (6–43 months)
Mean or median tumor size: 2.7 (1.0–4.4)
|
III
|
The data demonstrate that increasing tumor size is associated with poorer FN outcomes, however statistical analysis is lacking.
|
|
Goel et al, 1992
|
Retrospective case series, single institutional experience, including 42 patients who underwent RS craniotomy for attempted HP. All underwent RS craniotomy. FN function not reported.
Number of patients: 42
Mean or median follow-up: 2.5 years (among HP patients)
Mean or median tumor size: overall size not provided
|
III
|
Appears that size influenced hearing preservation based on data in table, but statistics were not performed.
|
|
Nissen et al, 1997
|
Retrospective case series, single institution, evaluating influence of 4 variables to FN outcome by review of 111 cases. Looked at tumor size, use of IOM, completeness of tumor resection, and surgical approach. TL approach in 47, 55 RS, and 9 combined cases.
>
Number of patients: 111
Mean or median follow-up: 12 months
Mean or median tumor size: no median, however most tumors <1.5 cm (51 patients).
|
III
|
FN function not found to be dependent on TL or RS approach.
Tumor size did not correlate FN functional outcome with no difference in significance. 90 patients with HB I or II function.
|
|
Nuseir et al, 2012
|
Retrospective case series, single institution evaluating management of VSs in elderly patients above 65 years old. TL approach mainly used.
Number of patients: 232
Mean or median follow-up: 12 months
Mean or median tumor size: 1.1-2 cm
|
III
|
Size of tumor did matter for FN injury and was significant.
|
|
Rachinger et al, 2011
|
Retrospective series, single institution, analyzing HP based on tumor size and preoperative hearing status. Patients operated on by RS approach.
Number of patients: 90
Mean or median follow-up: 12
Mean or median tumor size: 25 mm
|
III
|
Preoperative hearing and size of tumor impact HN preservation.
26 patients (29%) had HN preservation. Origin of tumor from SVN (42%) associated with HN preservation while 16% with IVN.
HB I/II in all patients.
|
|
Robinette et al, 1997
|
Retrospective, single institution, analyzing independent variable to tumor size. 31 (30%) patients with hearing preservation (postoperative PTA ≤85 dB HL)
Number of patients: 104
Mean or median follow-up: 15 months
Mean or median tumor size: 16.7 mm
|
III
|
Patients with small VSs ≤20 mm were significantly likely to have preserved auditory function after tumor removal than patients with larger tumors.
|
|
Roche et al, 2008
|
Retrospective, single institution study, analyzing FN function by TL approach.
Number of patients: 110
Mean or median follow-up: postoperatively only
Mean or median tumor size: Koos stage IV (>4 cm).
|
III
|
FN preservation in 60% of patients (HB 1 or 2) for large tumors. FN identified early in TL approach by reliable bony landmarks at fundus of IAC. Similar FN preservation between TL and RS approaches.
|
|
Samii et al, 2010
|
Retrospective, single institution study analyzing FN function preservation by size including giant VSs. >50 patients with giant VS >4 cm (group A); compared to group B with VS <3.9 cm (167 patients); RS used in all cases.
Number of patients: 50
Mean or median follow-up: not listed
Mean or median tumor size:
>Mean tumor size was 4.4 cm and 2.3 in group B
|
III
|
Total removal in all group A patients and 97.6% of group B patients; 75% of patients with giant VSs had excellent or good FN function and 91% of group B patients; 19% had fair function. Rate of patients with normal function was significantly different between groups A and B (25% compared with 63%).
33% of patients (3 patients) with good preoperative hearing level. HP is possible in giant VS patients. If useful hearing is present before surgery, then there is an 11% probability of its preservation.
Tumor size correlates with postoperative outcome.
|
|
Samii et al, 1991
|
Retrospective, single institution series examining intracanicular VSs resected by RS approach.
Number of patients: 16
Mean or median follow-up: 8 years
Mean or median tumor size: Intracanilicular only
|
III
|
100% FN preservation and function; 7 patients had good hearing preoperatively and were preserved.
Hearing preservation in 57%; HN preservation not better with IAC tumors in comparison to tumors extending out of the meatus.
|
|
Samii et al, 1992
|
Single, retrospective study analyzing VS resection in the elderly (> 65 years) by RS approach.
Number of patients: 61
Mean or median follow-up: Time of discharge from hospital.
Mean or median tumor size: 50% of tumors >3 cm.
|
III
|
69% had FN function that was good (I or II HB).
Serviceable hearing in 12%
|
|
Sepehmia et al, 2015
|
Single retrospective study of RS approach in 2 groups of patients with VSs ranging in size from 1–3 cm. >Patients with 1–2 cm (group A; 292 patients) in maximal diameter (intra and extrameatal diameter) and matched group of patients with VSs between 2–3 cm (154 patients) were assigned to group B.
Number of patients: 446
Mean or median follow-up: mean 67 months
Mean or median tumor size: 1–2 cm and 2–3 cm
|
III
|
HB I 94% in group A vs 78% group B. Preservation of preoperative hearing 51% group B vs 34% group A. Group A (1–2 cm) had higher FN preservation and HN preservation compared to GROUP B. Total removal should be performed at earliest stage.
|
|
Shamji et al, 2007
|
Retrospective single institution series analyzing TL approach and identification of preoperative clinical and intraoperative findings that predispose patients to FN dysfunction.
Number of patients: 128
Mean or median follow-up: 17 months
Mean or median tumor size:
2.3 cm (0.5–7 cm)
|
III
|
Small size and low intraoperative nerve stimulation <0.10 mA predictive of functional nerve preservation. 87% FN function preservation HB I and II. Tumor size not stratified.
|
|
Sharma et al, 2013
|
Retrospective single institution series analyzing patients with larger (≥3 cm) tumors resected by RS approach. Preoperative FN dysfunction correlated with poorer FN outcome. Patients with larger tumors and extrameatal growth correlated with poorer outcome.
Number of patients: 72
Mean or median follow-up: 1 year
Mean or median tumor size: ≥3 cm
|
III
|
Young patients and with tumors that are smaller experience a good FN outcome. Large extrameatal diameter of the tumor and tumor volume are associated with poor FN outcome. Grade 4–6 associated with size of 38.84 vs grade 1–3 in 33.28 cm.
|
|
Silverstein et al, 1993
|
Retrospective single institution series analyzing resection of VSs based on age over 65 and under 65. >For patients over 65, observation or subtotal resection is performed. 24 patients 65 or older. In patients under 65, HN preservation is attempted through RS in tumors <1.5 cm. 38 patients with TL approach. RS approach in 2 patients. TL approach for tumors of any size when hearing is not serviceable.
Number of patients: 130
Mean or median follow-up: 1 year
Mean or median tumor size: small (≤1.5 cm), medium (1.6–2.9 cm), and larger (≥3.0 cm).
|
III
|
Patients with tumors <1.5 cm with good hearing (hearing 30 dB and 70% speech discrimination) underwent RS approach. 47% HN preservation with tumors <2 cm.
58% of large tumors had FN preservation with TL. Subtotal excision resulted in 91% of patients with HB I or II.
|
|
Somers et al, 2001
|
Retrospective single institution series analyzing whether tumor size, extension into fundus, and intralabyrinthine signal intensity predict HN preservation with RS approach.
Number of patients: 26
Mean or median follow-up: 6 months
Mean or median tumor size: median of 15 mm where hearing was preserved (range 9–22 mm); 17 mm where hearing could not be preserved (17 mm; 5–28 mm).
|
III
|
Tumor size where hearing was preserved averaged 15 mm and 17 mm when not preserved.
HB I/II in 25 patients (96%).
|
|
Turel et al, 2014
|
Retrospective single institution series examining FN function after RS approach.
Number of patients: 100
Mean or median follow-up: 22 months
Mean or median tumor size: >mean size of 4.1 ± 0.8
|
III
|
Total excision in 89%. All underwent a RS approach; 77 out of the 100 patients had FN preservation with electrophysiological response; Good FN function in 40 patients (53%); With longer term follow-up, 44 patients with HB I/II (75%)
Early FN function in 75 patients: Tumor < 3 cm with I/II in 6/7 patients; 3-3.9 cm with I/II in 15/23 patients; ≥4 cm in 19/45 patients; long-term FN function in 59. larger size with less FN preservation
|
|
Van Dinther et al, 2011
|
Retrospective single institution series analyzing prognostic significance of FN function.
Number of patients: 123
Mean or median follow-up: 12 months
Mean or Median tumor size: >tumor size small (<10 mm), medium (10–25 mm), and large (>25 mm)
|
III
|
79.1% postoperatively with HB I/II deficit and 86% at 1 year
|
|
Zhang et al, 2005
|
Retrospective single institution series analyzing FN preservation in large VSs (>4 cm) by RS approach. FN preservation in 79.1% of patients.
Number of patients: 105
Mean or median follow-up: 12 months
Mean or Median tumor size:
>tumors ≥4 cm; 74 patients had a diameter <5.5 cm, and 31 had diameter >5.5 cm.
|
III
|
HB I/II in 57% at 1 year
|
ABR, auditory brainstem response; CPA, cerebellopontine angle; CSF, cerebrospinal fluid; FN, facial nerve; HB, House–Brackmann; HP, hearing preservation; IAC, internal auditory canal; IOM, intraoperative monitoring; MF, middle fossa; NP, not performed; RS, retrosigmoid; TL, translabyrinthine; VS, vestibular schwannoma.
Table 4. Evidence table for question 4
|
Author, Year
|
Study Description
|
Data Class
|
Conclusions
|
|
Samii et al, 1991
|
Retrospective review of 16 patients with purely IAC VSs, representing 2.7% of the author’s series during the reported interval of 8 years. FN and hearing results are presented.
|
III
|
The cochlear and FNs were preserved in all 16 cases, operated on by the RS approach. Facial function was normal in all patients. Hearing was preserved in 57% of patients, and dizziness resolved in all patients.
|
|
Yamakami et al. 2009
|
22 patients with small VSs—among which 6 were solely in the IAC—were reviewed with a view toward establishing rates of hearing preservation using combined ABR and CNAP monitoring.
|
III
|
Of 6 patients with IAC tumors operated on using ABR and CNAP monitoring, serviceable hearing (class A, B, or C) was preserved in 4/5 cases, with useful hearing (class A or B) in 3/5 patients.
|
|
Wang et al, 2013
|
Retrospective review of 103 patients with largely IAC tumors undergoing resection via MF approach followed for 5 years.
|
III
|
GTR rate was 98%. FN preservation rate (HB I/II) was 91%. The hearing preservation at 5 years was excellent; the initial AAO-HNS classification was preserved in 13 (65%) of the 20 patients who had class A hearing at 5 years, and in 8 (67%) of the 12 who had class B hearing at 5 years.
|
|
Pennings et al, 2011
|
Retrospective review of 47 patients with unilateral IAC VSs followed for a mean follow-up of 3.6 years. Growth, pure tone, and speech audiometry were recorded.
|
III
|
PTA thresholds and WRS deteriorated. PTA dropped by 13.4 dB, and WRS by 11.7% overall. 74% of patients with good hearing (by “50/50” rule) maintained this. 6 patients had large hearing losses, early in follow-up. Encouraging hearing rates were maintained with a watch and wait philosophy.
|
|
Roche et al, 2008
|
Forty-seven patients (22 men and 25 women) harboring an intracanalicular VS were followed prospectively. The mean follow-up period was 43.8 months (±40 months) ranging from 9–222 months.
|
III
|
Over the follow-up period, 76.6% tumors grew (mean 2.8 mm/year). 60% of patients did not change their hearing class during the study period. 37.5% patients experienced a >10-dB hearing loss, and 2 became deaf. Results from this study indicate that conservative management of IAC VSs exposes the patient to a significant risk of tumor growth and hearing loss.
|
|
Coletti et al, 2005
|
Prospective study of 70 well-matched VSs confined to the IAC ≤12 mm with class A or B hearing operated on by the middle fossa or RS approaches to compare hearing and FN outcomes.
|
II
|
VS size, IAC enlargement, and the distance from the IAC fundus were found to influence the postoperative results more than the type of approach itself. Facial function was nearly equal at 1 year with a trend to superior outcomes in the RS group. Hearing preservation rates were 57% in the RS group and 66% in the middle fossa group at 1 year, but were significantly improved in the middle fossa group when the distance from tumor to fundus in the IAC was <3 mm.
|
|
Kumon et al, 2000
|
Retrospective review of 15 intracanalicular VSs operated on by the middle fossa approach with attention to hearing and FN outcomes with mean follow-up of 45 months.
|
III
|
Hearing was preserved in 93% of patients, and serviceable hearing in 66%. Grade I or II facial function rates were 74%.
|
|
Haines et al. 1993
|
Retrospective review of hearing outcomes in 12 patients with intracanalicular VS operated on either through MF or posterior fossa approaches.
|
III
|
83% patients had grade I facial function postoperatively. Hearing preservation rates (GR grade I or II) rates were 82% overall. A trend toward improvement in hearing was noted in the MF patients.
|
|
Thomsen et al., 2000
|
Retrospective review of 40 unilateral IAC VSs followed over a period 3.6 years. There was a mean of 3.2 scans per patient.
|
III
|
67.5% of tumors grew. Of these, 55% showed progressive growth; 31% showed growth after a period of no growth; and 14% showed variable growth trajectory with an overall trend to growth. 13 patients underwent surgery, all of whom had grade I facial function at 1 year.
|
|
Shelton et al., 1991
|
Retrospective review of 39 patients with VS <0.5 cm in the IAC operated on through the MF approach.
|
III
|
Complete resection was achieved in 100% of patients. 97% of patients had grade I/II facial function at 1 year. 67.5% had measurable hearing after surgery; overall, 35% of patients had hearing similar to the preoperative level. Good hearing—with SRT ≤30 and SDS ≥70—was preserved in 46% patients.
|
|
Wigand et al, 1991
|
Retrospective review of 25 IAC VSs operated on by the middle fossa approach with reports on resection rates, FN outcome, and hearing function.
|
III
|
Complete resection was achieved in 100% of cases. FN outcomes were not divided by tumor size. Cochlear nerve was preserved in 100% of cases. Gross hearing preservation rates in IAC VSs were 71%. 48% patients with SRT ≤60 dB preserved this level after surgery.
|
|
Rowed et al, 1997
|
Retrospective review of 26 patients with IAC VSs operated on through a RS approach with a goal of hearing preservation.
|
III
|
GTR rates were at least 96%. 96% of patients had grade I or II facial function at follow-up. 50% of patients retained their preoperative level of hearing; patients had to have SRT ≤50 dB and SDS ≥60% to be considered hearing preservation candidates.
|
|
Stangerup, 2008
|
Over a 10-year period, 636 patients were prospectively allocated to a “wait and scan” management with magnetic resonance scanning and audiologic examination.
|
III
|
Only 17% of prospectively followed intracanalicular tumors grew after mean follow-up of approximately 4 years and 70% of patients who had 100% SDS at presentation still had class A hearing 10 years later.
|
AAO-HNS, American Academy of Otolaryngology-Head and Neck Surgery; CNAP, continuous noninvasive arterial pressure; FN, facial nerve; GTR, gross total resection; GR, Gardner–Robertson; HB, House–Brackmann; HP, hearing preservation; IAC, internal auditory canal; MF, middle fossa; PTA, pure tone average; RS, retrosigmoid; VS, vestibular schwannoma; WRS, word recognition score.
Table 5. Evidence table for question 5
|
Author, Year
|
Study Description
|
Data Class
|
Conclusions
|
|
Arriaga, 1997
|
Retrospective study, describing a series of patients in whom the HP surgical approach was individualized to patient and tumor characteristics.
|
III
|
N = 330 VSs, of which 60 (18%) procedures were done for HP. Preoperative HP surgery criteria was affected ear retained 50-dB SRT and 50% SDS. For patients electing HP surgery, tumor location and patient age directed surgical approach. MF was performed in 57% of patients, RS in 43%. Overall, measurable hearing was preserved in 77% of cases (MF, 85%; RS, 65%). Useful hearing (class A, B, or C) was preserved in 67% of cases (MF, 74%; RS, 58%). Hearing was preserved at the same or better class in 57% cases overall (68%, MF; 42%, RS).
|
|
Betchen, 2005
|
Retrospective review of 142 patients with RS describing long-term hearing results at 7 years in those patients who did have HP. One key point: they performed immediate postoperative audiograms on 35 patients. HP defined as preoperative PTA <50 dB and SDS >50%. Note: only 43/142 (30%) had HP; 38/42 had GR 1 or II hearing.
|
III
|
30/35 (85.7%) functional hearing preserved, 5/35 non-functional class III–IV GR. The results were independent of tumor size. These are for patients who did have HP in this study (only 30%). Also, 14.3% had delayed loss of functional hearing.
|
|
Brackmann, 1994
|
24 consecutive patients with MCF, HP rate described. Technical details described.
|
III
|
N = 24 consecutive MCF patients, average tumor size 1.1 cm. PTA, SDS. 71% retained hearing that was as good, better than, or almost as good as preoperative scores. 16% lost all hearing.
|
|
Brackmann, 2001
|
Retrospective review of patients with NF2 undergoing MCF. Early proactive management. N = 28 patients with 40 MCF procedures (some bilateral). Hearing and FN function reported.
|
III
|
Tumor size average: 1.1 cm. Measureable hearing preserved in 28 ears (70%), with 42.5% being within 15 dB PTA and 15% SDS of preoperative levels. 55% no change in hearing. 11 patients had bilateral MCF, and 82% retained some hearing bilaterally. Early surgical intervention to treat VSs in NF2 is feasible with high rates of HP.
|
|
Chee, 2003
|
Retrospective study describing HP in the early postoperative period and late postoperative period in small VSs (<2 cm), all RS. Serviceable hearing definition AAO-HNS
|
III
|
N = 126 <2 cm. 43/126 (34.1%) had HP, follow-up = 36 months. 40% of these 43 patients had deterioration over time. 30/43 had follow-up. Hearing preserved after VS surgery deteriorates at an accelerated pace as compared with the unoperated ear.
|
|
Colletti, 2003
|
Prospective study describing VS surgery between MCF and RS in HP.
|
III
|
Tumor size: 4–12 mm. 25 RS and 25 MF. Follow-up period: 12 months. The MF approach is commonly regarded as yielding better auditory results and poorer FN results compared with the RS-TM approach in VS surgery.
|
|
Colletti, 2005
|
Prospective study comparing MCF and RS approaches for intracanalicular VSs (size 4–12 mm).
|
III
|
N = 35 RS and 35 MCF. Pure IC VSs measuring 12 mm, PTA better than 50 dB hearing level, and speech discrimination score >50% (classes A and B AAO-HNS). HP A–C 57% RS, 66% MF. No statistical difference between technique, except a tendency of improved hearing in fundal tumors with the MCF.
|
|
Dornhoffer, 1995
|
Retrospective study, describing VSs via MCF. Useful hearing was defined as a minimum PTA of 500, 1000, and 2000 Hz of 50 dB and SDS of 50%.
|
III
|
N = 93 patients. Useful hearing preserved in 54 (58%). Near preoperative levels in 42 (45%). Hearing was preserved in 39 (60%) of 65 patients who had tumors that were less than or equal to 0.5 cm. Of the 11 patients who had tumors measuring between 0.5 cm to 1.0 cm of extension into the CPA, 7 (64%) exhibited HP. 17 patients had tumors that were larger than 1.0 cm of extension into the CPA, and hearing was preserved in only 8 (47%) of these patients.
|
|
Irving, 1998
|
Retrospective study evaluating HP surgery for VSs with the MCF and RS. 48 MF and 50 RS. AAO-HNS criteria: we generally consider patients with a PTA of more than 50 dB and an SDS of greater than 50% to be candidates for HP surgery.
|
III
|
Overall, 26 (52%) of the patients treated via the MF approach achieved a class B or better hearing result compared with seven (14%) of the RS group. Some hearing was preserved in 32 (64%) of the patients in the MF group and in 17 (34%) of the RS group. The results obtained by using the MF approach were
superior for intracanalicular tumors (P = .009, t-test), and for tumors with a CPA component measuring 0.1 to 1 cm (P = .006, t-test). An important issue in hearing conservation surgery is which patients should be considered candidates. Tumor size is the single factor consistently and strongly predictive of HP surgery outcome.
|
|
Harner, 2000
|
Retrospective study,
AAO-HNS hearing class.
|
III
|
721 VS procedures. Preoperative class A & B: 291 patients. Postoperative class A & B: 32.
|
|
Ginzkey, 2013
|
Retrospective study, on small tumors (mostly intracanalicular), using AAO and GR class. No NF2 patients, follow-up unclear
|
III
|
N = 89, 41/89 intracanalicular. 59/89 Stage 2. 65/89 class A & B, 74% had class A & B postoperative; GR 70/89 had class I/II, postop 70% had class I/II. The presented data underline the recommendation of early surgical treatment in small VSs as a valuable option for HP in the therapy of VSs.
|
|
Kanzaki, 1994
|
Retrospective chart review. HP using the EMCF. Among 69 cases of attempted HP, hearing was preserved in 17 (63%) of 27 cases with a tumor extending ≤3 mm from the IAC.
|
III
|
N = 69, 50/50, Hearing was preserved in 21 (81%) of 26 cases treated by the MCF approach and in 14 (33%) of 43 cases by the EMCF approach type III. The HP rate was significantly higher by the MCF approach than by the EMCF approach type Ill (P < .05).
|
|
Kutz, 2012
|
Retrospective chart review, describing the MCF and small VSs. N = 46. Of the 38 patients that had class A or B hearing preoperatively, 24 (63.2%) retained class A or B hearing and 29 (76.3%) retained class A, B, or C hearing.
|
III
|
When tumors were ≤10 mm in patients with class A or B preoperative hearing, 22 of 30 patients (73.3%) retained class A or B hearing. When the tumor size was >10 mm in patients with class A or B preoperative hearing, 2 of 8 patients (25%) retained class A or B hearing.
|
|
Lin, 2005
|
Retrospective chart review of patients treated with: 1) SRS, 2) RS, and 3) observation. HP at follow-up period are described. GR class.
|
III
|
N = 42 for SRS, N = 113 for RS, N = 86 for observation. Serviceable hearing preoperatively: SRS: 68%, RS 100%, observation 77.3%. Serviceable hearing after treatment: SRS: 6.7%, RS:15.9% and observation 33.3%. Of note, hearing acuity statistically worsened over the long term (P < .01) in all 3 groups.
|
|
Matthies, 2002
|
Retrospective chart review describing hearing outcomes for different size tumors. N = 1800; they used the Hannover Classification system.
|
III
|
In conclusion, preservation of auditory function may be achieved at increasing rates and quality and at more difficult conditions than previously anticipated. Despite reduced chances in medium and especially in large tumors with
brainstem involvement.
1.) There is a considerable number of patients with such tumors (T4) (36 % of the cases) and with retained (60%) and even with good preoperative hearing.
2.) There is no alternative to microsurgery for these large tumors.
3.) Auditory preservation is possible (20%) by refined microsurgical techniques accompanied by skillful neurophysiological monitoring.
4.) Chances are better (29%) in case of normal or good preoperative function.
5.) In selected cases, preservation will be achieved at the preoperative level (14% in patients with normal and 10% in patients with good preoperative function in T4 tumors).
|
|
Maw, 2003
|
Prospective study describing VS RS and HP.
|
III
|
N = 40 from a cohort of 191 that were followed. Used the AAO-HNS classification, GR, Shelton, and Sanna. Follow-up for minimum of 6 months.
Using appropriate surgical and monitoring techniques, it is possible to preserve useful hearing in approximately 50% of patients following removal of a VS via the RS approach.
|
|
Nonaka, 2013
|
Retrospective chart review, describing outcomes of a large skull base center. Surgical outcomes and complications were evaluated in a consecutive series of 410 unilateral VSs treated from 2000 to 2009. Clinical status and complications were assessed postoperatively (within 7 days) and at the time
of follow-up (range, 1–116 months; mean, 32.7 months).
|
III
|
Follow-up data were available for 357 of the 410 patients (87.1%). Microsurgical tumor resection was performed through a RS approach in 70.7% of cases. Thirty-three patients (8%) had intrameatal tumors and 204 (49.8%) had tumors that were 20 mm. Gross total resection was performed in 306 patients (74.6%). HP surgery was attempted in 170 patients with tumors 20 mm, and good hearing was preserved in 74.1%
|
|
Rigby, 1997
|
Retrospective chart review describing patient-perceived disability, not really hearing. To describe the long-term lifestyle consequences of VS removal from the patient’s perspective, patients filled out detailed questionnaires concerning their functional
status. The main outcome measures were the patient’s perception of his/her hearing, balance, facial expression, and eye function in relation to its impact upon the activities of daily life. A comparison of pretreatment with long-term posttreatment functional levels.
|
III
|
When asked to designate their “most significant” symptom, hearing loss was by far most prevalent (61.3%), followed by balance troubles (14.3%), and facial weakness (l0.1%). Both hearing in the tumor ear and overall auditory function (eg, the ability to understand in a restaurant) tended to worsen following surgery. One finding, which was both unanticipated and intriguing, was the improvement in sound localization ability reported by 57% of patients after surgery.
|
|
Sameshima, 2010
|
Retrospective chart review describing HP for MCF vs RS in tumors <1.5 cm. We reviewed 504 consecutive VS resections performed between November 1998 and September 2007 and identified 43 MF and 82 RS approaches for tumors smaller than 1.5 cm during HP surgery. Individual cases were examined postoperatively with respect to hearing ability, FN activity, operative time, blood loss, and symptoms resulting from retraction of the cerebellar or temporal lobes.
|
III
|
Good hearing function (AAO-HNS class B or better) was preserved in 76.7% of patients undergoing surgery via the MF approach and in 73.2% of the RS group (P = .9024).
|
|
Samii, 1995
|
Retrospective chart review, large case series
|
III
|
N = 900, where 653/900 had some hearing preoperatively. Hearing classification system is their own (see other column). The overall rate of HP was 38% (249 of 653), regardless of pre- and postoperative quality of hearing or of tumor sizes. In small tumor sizes (<3 cm) of diameter, preservation rate was 51 %, in large tumors (>3 cm) of diameter it was 22%. Study describes multiple hearing classification scales.
|
|
Samii, 2006
|
Retrospective chart review describing HP
|
III
|
N = 200 consecutive patients. In the patients with preserved hearing, the rate of anatomical preservation of the cochlear nerve was 84%. The overall rate of functional HP was 51%.
If only those patients with preoperative hearing are included in the analysis, the rate is 84%. The rate of preservation was highest (94%) among patients who had harbored a class T1 VS and gradually decreased as tumor extension increased: 89, 82, and 65% in classes T2, T3, and T4, respectively.
|
|
Sanna, 2004
|
Retrospective chart review describing HP between the MCF and RS, and using 2 hearing systems: modified Sanna and AAO-HNS.
|
III
|
AAO-HNS: HP rates of 62.7% in MCFA and 54.2% in RSA. Using the modified Sanna classification: a rate of 32.2% in MCFA and 31.3% in RSA. Thus, it fails to separate normal hearing and subnormal but socially serviceable hearing, increasing the chance of reporting a significant hearing deterioration as “not changed” or “preserved.” This classification frequently results in a false sense of success in HP when in fact, in most interventions near the cochlear nerve, the patient is left with at least a slight decrease that might shift hearing into the nonfunctional levels.
|
|
Sepehrnia, 2015
|
Retrospective chart review comparing 2 groups. Patients with VS sizes 1 to <2 cm in maximal intra-/extrameatal diameter (n = 292) were assigned to group “A” and a matched group of patients with VS between 2 and 3 cm in size (n = 154) were assigned to group “B.”
|
III
|
Significant differences in postoperative outcomes (P < .05) were found for FN function of HB grade I (94% group A vs 78% group B) and preservation of preoperative hearing (51% group B vs 34% group A). Even a small increase in tumor size correlated with a significant reduction in good hearing and facial preservation post-operatively, which implies that tumor removal should be performed at the earliest stage possible. Furthermore, these results contradict recommending the wait-and-see approach for intra-/extrameatal tumors.
|
|
Slattery, 1997
|
Prospective study describing VS MCF and HP. hearing level classified both by the AAO-HNS.
|
III
|
Measurable hearing was preserved in 68%, with 52% within 15 dB and 15% discrimination.
|
|
Slattery, 1998
|
Prospective study describing VS MCF and HP in NF2. Hearing level classified both by the AAO-HNS.
|
III
|
Eighteen patients diagnosed with NF2 underwent 23 middle fossa procedures. Measurable hearing was preserved in 65%, 48% within 15 dB of preoperative PTA and within 15% of preoperative speech discrimination. Bilateral
HP occurred in 5 patients.
|
|
Weber, 1996
|
Retrospective chart review, MCF and HP.
|
III
|
49 patients’ VSs were removed via the MCF approach. Hearing was preserved or improved in 69% of patients regardless of preoperative hearing levels.
|
|
Wiet, 2001
|
This retrospective study focuses on 2 outcome results after surgical intervention for
acoustic neuroma: (1) FN status, and (2) HP
|
III
|
484 patients with a VS; the overall success rate of retaining useful hearing was 27% (26 of 95). Class A hearing was retained
in 66% (10 of 15) of cases operated on through MF approach in the last 5 years
|
AAO-HNS, American Academy of Otolaryngology-Head and Neck Surgery; CPA, cerebellopontine angle; FN, facial nerve; GTR, gross total resection; GR, Gardner–Robertson; HB, House–Brackmann; HP, hearing preservation; IAC, internal auditory canal; MF, middle fossa; PTA, pure tone average; RS, retrosigmoid; SDS, speech discrimination score; TL, translabyrinthine; VS, vestibular schwannoma.
Table 6. Evidence table for question 6
|
Author, Year
|
Study Description
|
Data Class
|
Conclusions
|
|
Glasscock et al, 1992
|
Retrospective case series
1970–1990
52 tumors operated in 40 patients, various approaches
Mean size = 2.45 cm
|
III
|
43% useful HP
82% facial preservation (good facial outcome)
Resection (GTR, STR) status not reported
Recurrence rate = 5.8% (CT)
Mean follow-up: 5.4 years
The authors conclude while HP is realistic in sporadic tumors <2 cm, this cutoff should be 1.5 cm for NF2. Recommend operating on the smaller tumor with attempt at HP, then managing larger tumor based on postoperative hearing.
|
|
Samii et al, 1997
|
Retrospective case series (nonrandomized cohort study)
1978–1993
120 tumors operated in 82 patients, various approaches
38 tumors <3 cm, 82 tumors >3 cm
|
III
|
36% useful HP
85% FN preservation
Resection 88% GTR
Recurrence rate not reported
Follow-up not reported
The authors in this series compare their NF2 data to sporadic tumors. They conclude tumor progression is faster, the chances of anatomic and functional nerve preservation are lower, and the chances of good outcomes are best when surgery is performed early and when there is good preoperative hearing function, and the danger of sudden hearing loss is higher.
|
|
Brackmann et al, 2001
|
Retrospective case series
1988–1999
40 tumors operated in 28 patients, all MF approach
Mean size = 1.1 cm
|
III
|
60% useful HP
92% facial preservation (HB 1 or 2)
Resection: 100% GTR
Recurrence rate = NR
Mean follow-up = NR
The authors conclude that early middle fossa approach for HP provides good HP rates and great FN outcomes.
|
|
Slattery et al, 2007
|
Retrospective case series
1992–2004
47 tumors operated in 35 patients, all middle fossa approach
Mean size = 1.1 cm
|
III
|
48% useful HP
81% facial preservation (HB 1 or 2)
Resection: NR
Recurrence rate = NR
Mean follow-up = 2.8 years
The authors conclude that early MF approach for HP provides good HP rates and great FN outcomes. They encourage early surgery for these tumors.
|
|
Friedman et al, 2011
|
Retrospective case series
2000–2010
55 tumors operated in 37 patients, all MF approach
Mean size = 1.0 cm
|
III
|
50% useful HP
94% facial preservation (HB 1 or 2)
Resection: 96% GTR
Recurrence rate = 59% radiographic (MRI)
Mean follow-up = 37 months
The authors conclude that early middle fossa approach for HP provides good HP rates and great FN outcomes. They observe a high rate of tumor recurrence in the operative field that may affect long term HP.
|
|
Tysome et al, 2012
|
Retrospective case series
1981–2011
50 tumors operated in 44 patients, various approaches
Median size = 2.8 cm
|
III
|
Data unusable for HP
54% facial preservation (HB 1 or 2)
Resection: 78% GTR
Recurrence rate not reported
Follow-up not reported
The authors conclude that all tumors should be observed until evidence of growth on MRI. HP should be performed when able.
|
CT, computed tomography; FN, facial nerve; GTR, gross total resection; HB, House–Brackmann; HP, hearing preservation; IAC, internal auditory canal; MF, middle fossa; NF2, neurofibromatosis type 2; NR, not reported; STR, subtotal resection.
Table 7. Evidence table for question 7
|
Author, Year
|
Study Description
|
Data Class
|
Conclusions
|
|
Buchman et al, 1996
|
96 consecutive cases operated on by a combined neurosurgery/neurotology team were reviewed to assess the learning curve for achieving results similar to other experienced groups. In the majority of cases, a seasoned neurotologist was paired with 1 of 2 neurosurgeons.
|
III
|
The authors, a combined team, report superior rates of resection, reduced complications, and statistically significant improvement in FN outcomes with increasing case number, and that their learning curve was about 60 cases.
|
|
Fusco et al, 2014
|
706 surveys taken of residency-trained members of AANS to ascertain patterns of practice in the resection of VSs.
|
III
|
The majority of respondents (85.6%) treat VSs as part of an interdisciplinary team, while 75.8% of respondents feel this should be the “standard of care.” The survey did not include outcome measures.
|
|
Goodden et al, 2006
|
Survey of all neurosurgeons in the UK and Ireland treating VSs were analyzed to assess compliance with Clinical Effectiveness Guidelines outlined in 2002, specifically addressing teamwork during surgical resection.
|
III
|
75% of surgeons worked in conjunction with a specialist neuro-otolaryngologist.
Those who worked alone used a posterior fossa approach alone and operated on fewer cases.
|
|
Tonn et al, 2000
|
Retrospective review of 508 cases over 7 years to assess the benefits of a combined neurosurgery/ENT operative strategy using the retrosgimoid approach.
|
III
|
At 6 months, 88.7% of patients had grade I-III facial function; 38.9% of patients retained serviceable hearing. The surgeons emphasize the perceived value in working in a combined neurosourgery/neurotology team.
|
AANS, American Association of Neurological Surgeons; ENT, ear, nose, and throat; FN, facial nerve; United Kingdom; VS, vestibular schwannoma.
Table 8. Evidence table for question 8
|
Author, Year
|
Study Description
|
Data Class
|
Conclusions
|
|
Anaizi et al, 2014
|
Retrospective study of 76 patients with 78 VSs who underwent SRS after previous surgical resection.
|
III
|
Tumor control after SRS was achieved in 73 tumors (94%) at a median follow up of 43 months.
|
|
Pollock et al, 2008
|
Retrospective study of 50 VS patients who underwent SRS as adjuvant treatment for residual or recurrent tumors. Median follow-up was at least 3.5 years.
|
III
|
Tumor control rate was 96%. Useful (GR II) and residual hearing (GR III) remained unchanged in all patients who presented before SRS.
|
|
Brokinkel et al, 2014
|
Retrospective case series of 8 patients who received STR followed by GKRS for VSs.
|
III
|
At mean follow-up of 46 (12–73) months: 6 patients HB grade 1, 1 patient HB grade 2 (87.5% HB grade 1–2) and 1 patient HB grade 3. No tumors grew after GK and no new VIIth nerve weakness after GK.
|
|
Haque et al, 2011
|
Retrospective case series of 14 patients treated with surgery followed by SRS. Interval between surgery and SRS was 1–6 months (mean: 2.9 months).
|
III
|
At mean follow-up of 32 (12–72) months after SRS, 3 tumors grew after SRS, 1 required further surgery. 12 were HB grade 1–2 and 2 were HB grade 3 at last follow-up. They advocate for STR followed by SRS for large tumors.
|
|
Pan et al, 2012
|
Retrospective case series of 50 patients operated by a single surgeon. 9 had a GTR, 8 had a NTR, radical STR in 31 and STR in 2. (8 had STR plus GK). Radical STR was defined as >90% resection with small residual in IAC, FN, or brainstem.
|
III
|
At mean follow-up of 113 months (58–167), only 8/9 (89%) were recurrence free. 8/8 (100%) who had STR plus GK were recurrence free. FN function HB grade 1–2 of 56% in GTR, 62.5% in NTR, 87% in radical STR and 100% in STR. The authors do not explicitly say what the FN function was in the STR plus GK group of 8 patients.
|
|
Porter et al, 2013
|
Retrospective case series of 50 patients with large VSs who underwent STR plus GKRS.
|
III
|
Median follow-up of 33.8 months, 90% tumor control. HB grade 1–2 in 94%. They compared this to HB grade 1–2 of 27–58% with GTR of large VS in series in the literature (references 3, 22, 28, 38, 52, 62, and 65 in their bibliography).
|
|
Virk et al, 2014
|
Retrospective case series of 16 patients managed with STR for VSs. 6/16 had subsequent SRS.
|
III
|
After STR, 12 patients were HB grade 1–2, 2 patients were HB grade 3–4 and 2 patients were HB grade 5–6. 2 of 6 patients who had subsequent SRS deteriorated from HB grade 1–2 to HB grade 5–6 indicating radiation treatment can worsen FN function.
|
GKRS, Gamma Knife radiosurgery; GTR, gross total resection; HB, House–Brackmann; NTR, near total resection; SF-36, Short Form-36; STR, subtotal resection; VS, vestibular schwannoma.
Table 9. Evidence table for question 9
|
Author, Year
|
Study Description
|
Data Class
|
Conclusions
|
|
Pollock et al, 2006
|
Prospective cohort study comparing DHI scores between patients undergoing radiosurgery and microsurgery
|
II
|
Pretreatment DHI scores were similar between groups. Radiosurgery patients improved from a mean DHI of 11.0 to a mean of 8.4 after treatment. Microsurgery patients worsened from a mean DHI of 11.9 to a mean of 16.5 after treatment.
|
|
Myrseth et al, 2009
|
Prospective cohort comparing balance outcomes between patients treated with radiosurgery or microsurgery
|
II
|
There was no significant difference in balance outcomes between the radiosurgery or microsurgery groups.
|
|
Stavas et al, 2014
|
Prospective observation
|
III
|
10 patients included, no statistically significant associations or identifiable trends between radiation dose and change in vestibular function or DHI scores found. Radiation dose to the vestibule does not reliably predict change in objective or subjective vestibular outcome measures.
|
|
Varughese et al, 2012
|
Prospective observation
|
III
|
193 patients with VSs given conservative management. Treatment did not affect QOL or symptoms. Vestibular complaints improved slightly.
|
|
Humphriss et al, 2004
|
Retrospective case review
|
III
|
Looked at incidence of dizziness handicap after VS resection in 100 patients. Dizziness does not get worse after surgery for most patients. In those where it does, it gets worse before 3 months postoperatively. No change postoperatively in 73, significantly worse in 21 postoperatively.
|
|
Kane, 1995
|
Retrospective case series of 56 patients undergoing surgery for VSs, including 10 with preoperative significant balance dysfunction
|
III
|
40% of 10 patients with preoperative balance problems improved after surgery, 12.5% of previously unimpaired patients developed new problems after surgery.
|
|
Rigby, 1997
|
Retrospective case series of 200 patients undergoing surgery for VSs
|
III
|
Of the 100 patients without preoperative dysfunction, 30% developed this after surgery. There was no comment regarding the rates of improvement in patients with preoperative dysfunction.
|
|
Andersson, 1997
|
Retrospective case review involving patients undergoing surgery tested with a subjective assessment of balance
|
III
|
52/82 (63%) of patients with preoperative balance problems improved after surgery.
|
|
Driscoll, 1998
|
Retrospective case series involving 115 patients undergoing surgery with preoperative balance dysfunction
|
III
|
73/115 (63%) of patients with preoperative balance problems improved after surgery.
|
|
Karpinos et al, 2002
|
Retrospective case review comparing balance outcomes between 47 patients treated with radiosurgery and 15 patients treated with microsurgery.
|
III
|
7/43 (14%) of radiosurgery patients and 4/15 (27%) patients treated with microsurgery with preoperative balance problems improved after treatment. This was not a statistically significant difference.
|
|
Timmer et al, 2010
|
Retrospective case review
|
III
|
Survey of 108 VS patients (97 included in study). SF-36 scores showed results comparable to those for a normal Dutch population. GBI showed a marginal decline in QOL. No correlation was found between QOL and sex, age, tumor size, or radiation dose. Increased audiovestibular symptoms after GKRS were correlated with a decreased GBI score. Decreased symptoms were correlated with a higher QOL post-GKRS.
|
|
Feigl et al, 2011
|
Retrospective case review
|
III
|
92 patients analyzed (preoperative symptoms in 53 [40 at grade 2 symptoms], postoperative symptoms in 90 [71 at grade 2 symptoms]).
|
|
Wagner et al, 2011
|
Retrospective case review
|
III
|
38 patients included (22 = MS, 16 = RS), loss of vestibular function in VSs clearly correlates with tumor size. However, loss of vestibular function was not strictly associated with a long-term deterioration of quality of life. Hearing was significantly influenced by the size of the VS and the manner of treatment.
|
|
Carlson et al, 2014
|
Retrospective comparative
|
III
|
Ongoing dizziness and headache are the strongest predictors of long-term quality-of-life reduction in patients with sporadic VSs, while the impact of hearing loss, FN function, and tinnitus are less by comparison. In another survey paper: 8 years following treatment, over half of patients with VSs reported ongoing dizziness. Female sex, older age, larger tumor size, preexisting diagnosis of headache or migraine, and symptoms of dizziness predating treatment may help predict the risk of lasting dizziness in VS patients. Treatment modality (stereotactic, microsurgery, observation) does not appear to influence long-term DHI score.
|
|
Al-Shudifat et al, 2014
|
Retrospective case review
|
III
|
430 questionnaires sent (93% return rate) to identify predictive factors for outcomes following surgery for VS, patients divided into two age groups (< 64 years, ≥ 64 years) to assess either WC or ILS. In the group <64 years, age, sex, and tumor diameter were independent predictive factors for postoperative WC using multivariate analysis (high risk, reduced WC surgical patient = female older than 50 with large tumor (>25 mm). In addition, the SF-36 did not correlate to the WC and ILS outcome measures.
|
|
Robinett et al, 2014
|
Retrospective Case-control
|
III
|
PANQOL survey sent to 600 VS patients (49% return rate), only significant difference in composite QOL occurred between 0–5 years (stereotactic radiation scores better than both microsurgery and observation treatment methods), no statistically significant QOL differences at >5 years.
|
DHI, dizziness handicap index; FN, facial nerve; GBI, Glasgow Benefit Inventory; GKRS, Gamma Knife radiosurgery; ILS, independent life status; PANQOL, Penn Acoustic Neuroma Quality of Life; QOL, quality of life; SF-36, Short Form-36; VS, vestibular schwannoma; WC, work capacity.
Table 10. Evidence table for question 10
|
Author, Year
|
Study Description
|
Data Class
|
Conclusions
|
|
Badakhshi et al, 2014
|
Retrospective review of 250 cases of VSs treated with SRS or FSRT; 61 patients with trigeminal symptoms preoperatively: 34 patients with trigeminal pain, 27 with dysesthesia.
|
III
|
10/61(16.3%) with relief of trigeminal symptoms after radiotherapy.
|
|
Squire et al, 2012
|
Retrospective review of 21 patients with facial pain and intracranial neoplasms treated with GKRS; 5/21 with VS, evaluated using BNI criteria.
|
III
|
4/5 (80%) patients with VS experienced a treatment response at 6 months (BNI score I–III for facial pain). Freedom from BNI IV–V in all patients not separated by tumor type in 66% of patients at 1 year and 53% of patients at 2 years.
|
|
Barker et al, 1996
|
Retrospective case series of posterior fossa tumors presenting with trigeminal neuralgia patients. 26 patients identified with trigeminal symptoms and posterior fossa tumors of which 8 were caused by VSs.
|
III
|
7/8 patients with VS had improvement in symptoms. Vascular compression by artery or vein was identified in majority of cases (23/26).
|
|
Samii et al, 1995
|
Retrospective review of 9 patients with small acoustic neurinomas not reaching the brainstem causing trigeminal neuralgia; patients treated with suboccipital craniotomy and tumor resection, vascular decompression if any compression identified.
|
III
|
In 9/9 patients, a co-existing vascular compression was identified, 9/9 with immediate pain relief, and 9/9 without recurrence at 6-month follow-up.
|
|
Puca et al, 1995
|
Retrospective review of 136 patients with extraxial masses treated with surgery; 88 acoustic neurinomas, 21 sphenopetrosal meningiomas, 11 CPA meningiomas, 10 CPA dermoids, 1 trigeminal neurinoma, 5 misc tumors. Trigeminal symptoms in 33% (9 with typical trigeminal neuralgia).
|
III
|
28.4% (25/88) of acoustic neurinomas had trigeminal symptoms, 3/88 had typical trigeminal neuralgia; outcomes not reported based on type of tumor, but 8/9 patients with typical trigeminal neuralgia had improvement in pain symptoms.
|
BNI, Barrow Neurological Institute; CPA, cerebellopontine angle; FSRT, fractionated stereotactic radiotherapy; GKRS, Gamma Knife radiosurgery; VS, vestibular schwannoma.
Table 11. Evidence table for question 11
|
Author, Year
|
Study Description
|
Data Class
|
Conclusions
|
|
Lee et al, 2014
|
Retrospective case series adding 6 more patients for total of 13 to previous report of tumors operated after GKRS.
|
III
|
1/13 was MPNST. “Remaining 12 normal FN function” at median follow-up of 71.3 months (13.4–205.5 months). All STR.
|
|
Hong et al, 2014
|
Retrospective case series comparing 15 patients who had surgery after previous surgery to 5 patients who had surgery after previous radiation.
|
III
|
In the 5 patients with surgery after radiation: 3 started out HB 1–2, 2 started out HB gr 3–4. At last follow-up (mean 28 months) 1 patient was dead, 1 remained HB grade 3 unchanged from preoperatively and 3 were HB grade 1–2. 3/5 had GTR.
|
|
Gerganov et al, 2012
|
Retrospective case series examining 15 patients with previous radiation then surgery (group A); 13 patients previous surgery, then radiation then surgery again (group B) and 30 patients with no previous radiation (group C) that served as a control group.
|
III
|
Patients who had prior radiation had a higher risk of postoperative hematoma. Anatomic FN preservation was better in group C (93.3%) vs group A (86.7%). HB grade 1–2 was 8/14 (57%) in group A and 21/30 (70%) in group C. Patients without radiation did better compared to patients with previous radiation then surgery. (Patients who had previous radiation and previous surgery – group B had worse outcomes.)
|
|
Friedman et al, 2011
|
Retrospective case series of 73 patients operated after previous radiation of a variety of types.
|
III
|
58 (79.5%) underwent GTR. VIIth nerve was anatomically lost in 10 (13.7%). Of patients who started with HB grade 1–2, 65% maintained HB grade 1–2. Patients that underwent STR in this cohort had better VIIth nerve outcomes.
|
|
Lee et al, 2010
|
Retrospective case series of 7 patients operated after GKRS.
|
III
|
All had STR. “The authors did not think radiosurgical treated tumors were more difficult to remove.” 6/7 patients with symptomatic improvement after surgery; 1/7 complete facial palsy in a MPNST.
|
|
Liscak et al, 2009
|
Retrospective case control of 351 patients who underwent GKRS including 5 patients who ended up having surgery after SRS. 3/5 had previously also had prior microsurgery, one of them twice. Preoperative HB grade 1–3.
|
III
|
All had GTR. 1/5 had HB grade 4 postoperatively and 4/5 HB grade 6 indicating bad FN outcomes in postradiated tumors that grow and receive GTR.
|
|
Shuto et al, 2008
|
Retrospective case series of 12 patients operated after SRS.
|
III
|
8/12 (66%) started out HB grade 1, and 5 remained HB grade 1 and 3 ended up HB grade 3–4. All STR. “The surgeons felt that complete dissection of the FN and tumor was difficult in most operations because of severe adhesions or color change.”
|
|
Pollock, 2006
|
Retrospective case series of 208 patients receiving GKRS.
|
III
|
5 patients underwent surgery after prior GKRS. 2 had GTR, and 3 had NTR. In 1 of the GTR cases the VIIth nerve was severed. The 2 patients who had GTR after GKRS had complete facial palsies, and the 3 with less than GTR preserved good facial movements; therefore, recommends less than GTR if surgery recommended after failed GKRS.
|
|
Friedman et al, 2005
|
Retrospective case control series of 38 patients with previous radiation of a variety of types followed by surgical resection compared to a similar cohort of size-matched nonirradiated tumors.
|
III
|
The authors conclude: 1. FN more adherent (89% vs 63% of the time). 2. GTR lower in radiated group (78.9 vs 97.4%). 3. At 1-year postoperatively, HB grade 1–2 lower in radiated group (37% vs 70%) and bad FN outcome (HB grade 5–6) was higher (50% vs 18%) for radiated cohort. Therefore, surgery was deemed more difficult after radiation.
|
|
Pollock et al, 1998
|
Retrospective case series of 13 patients who underwent delayed microsurgery after radiosurgery at two centers. 6/13 also had previous microsurgery.
|
III
|
7/13 had a GTR, 4/13 had a NTR and 2/13 had a STR. Anatomic VIIth nerve preservation in 10/13. Preoperatively, 11 patients were HB grade 1, 1 HB grade 4, and 1 HB grade 6. At median follow-up of 18 months (3–67 months), 3 patients were HB grade 1–2, 3 patients were HB grade 3–4, and 7 were HB grade 6. 1 patient had a brainstem infarct. “The operating surgeons indicated that in comparison with their experience in VS patients who had not undergone radiosurgery, the tumor was more difficult to resect in 8 patients, no different in 4 patients, and easier in 1 patient.”
|
FN, facial nerve; FSRT, fractionated stereotactic radiotherapy; GKRS, Gamma Knife radiosurgery; GTR, gross total resection; HB, House–Brackmann; MPNST, malignant peripheral nerve sheath tumor; NTR, near total resection; STR, subtotal resection; VS, vestibular schwannoma.
References
1. Mahboubi H, Maducdoc MM, Yau AY, et al. Vestibular Schwannoma excision in sporadic versus neurofibromatosis type 2 populations. Otolaryngol Head Neck Surg 2015;153(5):822-831.
2. Cushing H. Tumors of the nervus acusticus and the syndrome of the cerebellopontine angle. Philadelphia: W. B. Saunders Co.; 1917.
3. Cushing H. Further concerning the acoustic neuromas. Laryngoscope 1921;31(4):209-228.
4. Dandy W. An operation for the total extirpation of tumors of the cerebellopontine angle. A preliminary report. Bull Johns Hopkins Hosp 1922;33:344-345.
5. Dandy W. An operation for the total removal of cerebellopontine (acoustic) tumors. Surg Gynecol Obstet 1925;41:129-148.
6. Kastner M, Wilczynski NL, Walker-Dilks C, McKibbon KA, Haynes B. Age-specific search strategies for Medline. J Med Internet Res 2006;8(4):e25.
7. Haynes RB, McKibbon KA, Wilczynski NL, Walter SD, Werre SR, Hedges T. Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. BMJ 2005;330(7501):1179.
8. Montori VM, Wilczynski NL, Morgan D, Haynes RB, Hedges T. Optimal search strategies for retrieving systematic reviews from Medline: analytical survey. BMJ 2005;330(7482):68.
9. Wong SS, Wilczynski NL, Haynes RB. Comparison of top-performing search strategies for detecting clinically sound treatment studies and systematic reviews in MEDLINE and EMBASE. J Med Library Assoc 2006;94(4):451-455.
10. Zhang L, Ajiferuke I, Sampson M. Optimizing search strategies to identify randomized controlled trials in MEDLINE. BMC Med Res Methodol 2006;6:23.
11. Topfer LA, Parada A, Menon D, Noorani H, Perras C, Serra-Prat M. Comparison of literature searches on quality and costs for health technology assessment using the MEDLINE and EMBASE databases. Int J Technol Assess Health Care 1999;15(2):297-303.
12. Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound prognostic studies in MEDLINE: an analytic survey. BMC Med 2004;2:23.
13. Wilczynski NL, Haynes RB, Hedges T. EMBASE search strategies achieved high sensitivity and specificity for retrieving methodologically sound systematic reviews. J Clin Epidemiol 2007;60(1):29-33.
14. Hillman T, Chen DA, Arriaga MA, Quigley M. Facial nerve function and hearing preservation acoustic tumor surgery: does the approach matter? Otolaryngol Head Neck Surg 2010;142(1):115-119.
15. Meyer TA, Canty PA, Wilkinson EP, Hansen MR, Rubinstein JT, Gantz BJ. Small acoustic neuromas: surgical outcomes versus observation or radiation. Otol Neurotol 2006;27(3):380-392.
16. Kanzaki J, Ogawa K, Inoue Y, Shiobara R. Hearing preservation surgery in acoustic neuroma patients with normal hearing. Skull Base Surg 1997;7(3):109-113.
17. Nonaka Y, Fukushima T, Watanabe K, et al. Contemporary surgical management of vestibular schwannomas: analysis of complications and lessons learned over the past decade. Neurosurgery 2013;72(2 suppl operative):ons103-ons115.
18. Rabelo de Freitas M, Russo A, Sequino G, Piccirillo E, Sanna M. Analysis of hearing preservation and facial nerve function for patients undergoing vestibular schwannoma surgery: the middle cranial fossa approach versus the retrosigmoid approach—personal experience and literature review. Audiol Neurootol 2012;17(2):71-81.
19. Sameshima T, Fukushima T, McElveen Hr JT, Friedman AH. Critical assessment of operative approaches for hearing preservation in small acoustic neuroma surgery: retrosigmoid vs middle fossa approach. Neurosurgery 2010;67(3):640-644.
20. Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): the facial nerve—preservation and restitution of function. Neurosurgery 1997;40(4):684-694.
21. Yang J, Grayeli AB, Barylyak R, Elgarem H. Functional outcome of retrosigmoid approach in vestibular schwannoma surgery. Acta Otolaryngol 2008;128(8):881-886.
22. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg 1985;93(2):146-147.
23. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere's disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc. Otolaryngol Head Neck Surg 1995;113(3):181-185.
24. Baumann I, Polligkeit J, Blumenstock G, Mauz PS, Zalaman IM, Maassen MM. Quality of life after unilateral acoustic neuroma surgery via middle cranial fossa approach. Acta Otolaryngol 2005;125(6):585-591.
25. Chovanec M, Zverina E, Profant O. Does attempt at hearing preservation microsurgery of vestibular schwannoma affect postoperative tinnitus? 2015;2015:783169.
26. Tos M, Charabi S, Thomsen J. Clinical experience with vestibular schwannomas: epidemiology, symptomatology, diagnosis, and surgical results. Eur Arch Otorhinolaryngol 1998;255(1):1-6.
27. Fenton JE, Chin RY, Fagan PA, Sterkers O, Sterkers JM. Predictive factors of long-term facial nerve function after vestibular schwannoma surgery. Otol Neurotol 2002;23(3):388-392.
28. Dunn IF, Bi WL, Erkmen K, et al. Medial acoustic neuromas: clinical and surgical implications. J Neurosurg 2014;120(5):1095-1104.
29. Moffat DA, Parker RA, Hardy DG, Macfarlane R. Factors affecting final facial nerve outcome following vestibular schwannoma surgery. J Laryngol Otol 2014;128(5):406-415.
30. Haque R, Wojtasiewicz TJ, Gigante PR, et al. Efficacy of facial nerve-sparing approach in patients with vestibular schwannomas. J Neurosurg 2011;115(5):917-923.
31. Misra BK, Purandare HR, Ved RS, Bagdia AA, Mare PB. Current treatment strategy in the management of vestibular schwannoma. Neurol India 2009;57(3):257-263.
32. Bae CW, Cho YH, Hong SH, Kim JH, Lee JK, Kim CJ. The anatomical location and course of the facial nerve in vestibular schwannomas: a study of 163 surgically treated cases. J Korean Neurosurg Soc 2007;42(6):450-454.
33. Mirzayan MJ, Gerganov VM, Ludemann W, Oi S, Samii M, Samii A. Management of vestibular schwannomas in young patients-comparison of clinical features and outcome with adult patients. Childs Nerv Syst 2007;23(8):891-895.
34. Darrouzet V, Martel J, Enee V, Bebear JP, Guerin J. Vestibular schwannoma surgery outcomes: our multidisciplinary experience in 400 cases over 17 years. Laryngoscope 2004;114(4):681-688.
35. Couloigner V, Gervaz E, Kalamarides M, et al. Clinical and histologic parameters correlated with facial nerve function after vestibular schwannoma surgery. Skull Base 2003;13(1):13-19.
36. Mamikoglu B, Wiet RJ, Esquivel CR. Translabyrinthine approach for the management of large and giant vestibular schwannomas. Otol Neurotol 2002;23(2):224-227.
37. Guerin C, Sampath P, Long DM. Acoustic neuroma: outcome of surgical resection and study on the anatomy of facial and cochlear nerves. Ann Acad Med Singapore 1999;28(3):402-408.
38. Lanman TH, Brackmann DE, Hitselberger WE, Subin B. Report of 190 consecutive cases of large acoustic tumors (vestibular schwannoma) removed via the translabyrinthine approach. J Neurosurg 1999;90(4):617-623.
39. Andersson G, Ekvall L, Kinnefors A, Nyberg G, Rask-Andersen H. Evaluation of quality of life and symptoms after translabyrinthine acoustic neuroma surgery. Am J Otol 1997;18(4):421-426.
40. Colletti V, Fiorino F, Mocella S, Carner M, Policante Z. “En-bloc” removal of small- to medium-sized acoustic neuromas with retrosigmoid-transmeatal approach. Skull Base Surg 1997;7(1):31-38.
41. Grey PL, Moffat DA, Palmer CR, Hardy DG, Baguley DM. Factors which influence the facial nerve outcome in vestibular schwannoma surgery. Clin Otolaryngol Allied Sci 1996;21(5):409-413.
42. Lalwani AK, Butt FY, Jackler RK, Pitts LH, Yingling CD. Facial nerve outcome after acoustic neuroma surgery: a study from the era of cranial nerve monitoring. Otolaryngol Head Neck Surg 1994;111(5):561-570.
43. Nissen AJ, Sikand A, Welsh JE, Curto FS, Gardi J. A multifactorial analysis of facial nerve results in surgery for cerebellopontine angle tumors. Ear Nose Throat J 1997;76(1):37-40.
44. Sterkers JM, Morrison GA, Sterkers O, El-Dine MM. Preservation of facial, cochlear, and other nerve functions in acoustic neuroma treatment. Otolaryngol Head Neck Surg 1994;110(2):146-155.
45. Van Abel KM, Carlson ML, Driscoll CL, Neff BA, Link MJ. Vestibular schwannoma surgery in the elderly: a matched cohort study. J Neurosurg 2014;120(1):207-217.
46. Zhang Z, Wang Z, Huang Q, Yang J, Wu H. Removal of large or giant sporadic vestibular schwannomas via translabyrinthine approach: a report of 115 cases. ORL J Otorhinolaryngol Relat Spec 2012;74(5):271-277.
47. Arnoldner C, Mick P, Pirouzmand F, et al. Facial nerve prognostication in vestibular schwannoma surgery: the concept of percent maximum and its predictability. Laryngoscope 2013;123(10):2533-2538.
48. Esquia-Medina GN, Grayeli AB, Ferrary E, et al. Do facial nerve displacement pattern and tumor adhesion influence the facial nerve outcome in vestibular schwannoma surgery? Otol Neurotol 2009;30(3):392-397.
49. Gerganov VM, Klinge PM, Nouri M, Stieglitz L, Samii M, Samii A. Prognostic clinical and radiological parameters for immediate facial nerve function following vestibular schwannoma surgery. Acta Neurochir 2009;151(6):581-587.
50. Kim AH, Edwards BM, Telian SA, Kileny PR, Arts HA. Transient evoked otoacoustic emissions pattern as a prognostic indicator for hearing preservation in acoustic neuroma surgery. Otol Neurotol 2006;27(3):372-379.
51. Kobayashi M, Tsunoda A, Komatsuzaki A, Yamada I. Distance from acoustic neuroma to fundus and a postoperative facial palsy. Laryngoscope 2002;112(1):168-171.
52. Matthies C, Samii M. Vestibular schwannomas and auditory function: options in large T3 and T4 tumors? Neurochirurgie 2002;48(6):461-470.
53. Ferber-Viart C, Laoust L, Boulud B, Duclaux R, Dubreuil C. Acuteness of preoperative factors to predict hearing preservation in acoustic neuroma surgery. Laryngoscope 2000;110(1):145-150.
54. Hahn A, Fundova P, Schneider D. Audiovestibular findings prior to and after acoustic neuroma surgery. Int Tinnitus J 2000;6(1):67-69.
55. Colletti V, Fiorino FG, Sacchetto L. Iatrogenic impairment of hearing during surgery for acoustic neuroma. Skull Base Surg 1996;6(3):153-161.
56. Leonetti JP. The diagnosis and management of acoustic neuromas: contemporary practice guidelines. Compr Ther 1995;21(2):68-73.
57. Kanzaki J, O-Uchi T, Ogawa K, Shiobara R, Toya S. Hearing preservation by the extended and nonextended middle cranial fossa approach for acoustic neuroma. Skull Base Surg 1994;4(2):76-81.
58. Arriaga MA, Long S, Nelson R. Clinical correlates of acoustic neuroma volume. Am J Otol 1993;14(5):465-468.
59. Berges C, Fraysse B, Yardeni E, Rugiu G. Intraoperative facial nerve monitoring in posterior fossa surgery: prognostic value. Skull Base Surg 1993;3(4):214-216.
60. Kirkpatrick PJ, Tierney P, Gleeson MJ, Strong AJ. Acoustic tumour volume and the prediction of facial nerve functional outcome from intraoperative monitoring. Br J Neurosurg 1993;7(6):657-664.
61. Goel A, Sekhar LN, Langheinrich W, Kamerer D, Hirsch B. Late course of preserved hearing and tinnitus after acoustic neurilemoma surgery. J Neurosurg 1992;77(5):685-689.
62. Nuseir A, Sequino G, De Donato G, Taibah A, Sanna M. Surgical management of vestibular schwannoma in elderly patients. Eur Arch Otorhinolaryngol 2012;269(1):17-23.
63. Rachinger J, Rampp S, Prell J, Scheller C, Alfieri A, Strauss C. Tumor origin and hearing preservation in vestibular schwannoma surgery. J Neurosurg 2011;115(5):900-905.
64. Robinette MS, Bauch CD, Olsen WO, Harner SG, Beatty CW. Nonsurgical factors predictive of postoperative hearing for patients with vestibular schwannoma. Am J Otol 1997;18(6):738-745.
65. Roche PH, Pellet W, Moriyama T, Thomassin JM. Translabyrinthine approach for vestibular schwannomas: operative technique. Prog Neurol Surgery 2008;21:73-78.
66. Samii M, Gerganov VM, Samii A. Functional outcome after complete surgical removal of giant vestibular schwannomas. J Neurosurg 2010;112(4):860-867.
67. Samii M, Matthies C, Tatagiba M. Intracanalicular acoustic neurinomas. Neurosurgery 1991;29(2):189-198.
68. Samii M, Tatagiba M, Matthies C. Acoustic neurinoma in the elderly: factors predictive of postoperative outcome. Neurosurgery 1992;31(4):615-619.
69. Sepehrnia A, Borghei-Razavi H. Vestibular schwannoma between 1 and 3 cm: importance of the tumor size in surgical and functional outcome. Clin Neurol Neurosurg 2015;129:21-26.
70. Shamji MF, Schramm DR, Benoit BG. Clinical predictors of facial nerve outcome after translabyrinthine resection of acoustic neuromas. Clin Invest Med 2007;30(6):E233-E239.
71. Sharma M, Sonig A, Ambekar S, Nanda A. Radiological and clinical factors predicting the facial nerve outcome following retrosigmoid approach for large vestibular schwannomas (VSs). J Neurol Surg B Skull Base 2013;74(5):317-323.
72. Silverstein H, Rosenberg SI, Flanzer JM, Wanamaker HH, Seidman MD. An algorithm for the management of acoustic neuromas regarding age, hearing, tumor size, and symptoms. Otolaryngol Head Neck Surg 1993;108(1):1-10.
73. Somers T, Casselman J, de Ceulaer G, Govaerts P, Offeciers E. Prognostic value of magnetic resonance imaging findings in hearing preservation surgery for vestibular schwannoma. Otol Neurotol 2001;22(1):87-94.
74. Turel MK, Babu KS, Singh G, Chacko AG. The utility of facial nerve amplitude and latency ratios in predicting postoperative facial nerve function after vestibular schwannoma surgery. Neurol India 2014;62(2):178-182.
75. van Dinther JJ, Van Rompaey V, Somers T, Zarowski A, Offeciers FE. Prognostic significance of electrophysiological tests for facial nerve outcome in vestibular schwannoma surgery. B-ENT 2011;7(2):115-119.
76. Zhang X, Fei Z, Chen YJ, et al. Facial nerve function after excision of large acoustic neuromas via the suboccipital retrosigmoid approach. J Clin Neurosci 2005;12(4):405-408.
77. Yamakami I, Yoshinori H, Saeki N, Wada M, Oka N. Hearing preservation and intraoperative auditory brainstem response and cochlear nerve compound action potential monitoring in the removal of small acoustic neurinoma via the retrosigmoid approach. J Neurol Neurosurg Psychiatry 2009;80(2):218-227.
78. Wang AC, Chinn SB, Than KD, et al. Durability of hearing preservation after microsurgical treatment of vestibular schwannoma using the middle cranial fossa approach. J Neurosurg 2013;119(1):131-138.
79. Pennings RJ, Morris DP, Clarke L, Allen S, Walling S, Bance ML. Natural history of hearing deterioration in intracanalicular vestibular schwannoma. Neurosurgery 2011;68(1):68-77.
80. Roche PH, Soumare O, Thomassin JM, Regis J. The wait and see strategy for intracanalicular vestibular schwannomas. Prog Neurol Surg 2008;21:83-88.
81. Colletti V, Fiorino F. Is the middle fossa approach the treatment of choice for intracanalicular vestibular schwannoma? Otolaryngol Head Neck Surg 2005;132(3):459-466.
82. Kumon Y, Sakaki S, Kohno K, et al. Selection of surgical approaches for small acoustic neurinomas. Surg Neurol 2000;53(1):52-59.
83. Haines SJ, Levine SC. Intracanalicular acoustic neuroma: early surgery for preservation of hearing. J Neurosurg 1993;79(4):515-520.
84. Thomsen J, Charabi S, Tos M, Mantoni M, Charabi B. Intracanalicular vestibular schwannoma—therapeutic options. Acta Otolaryngol Suppl 2000;543:38-40.
85. Shelton C, Hitselberger WE. The treatment of small acoustic tumors: now or later? Laryngoscope 1991;101(9):925-928.
86. Wigand ME, Haid T, Berg M, Schuster B, Goertzen W. Extended middle cranial fossa approach for acoustic neuroma surgery. Skull Base Surg 1991;1(3):183-187.
87. Rowed DW, Nedzelski JM. Hearing preservation in the removal of intracanalicular acoustic neuromas via the retrosigmoid approach. J Neurosurg 1997;86(3):456-461.
88. Stangerup SE, Caye-Thomasen P, Tos M, Thomsen J. Change in hearing during “wait and scan” management of patients with vestibular schwannoma. J Laryngol Otol 2008;122(7):673-681.
89. Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC. Otolaryngol Head Neck Surg 1995;113(3):179-180.
90. Arriaga MA, Chen DA, Fukushima T. Individualizing hearing preservation in acoustic neuroma surgery. Laryngoscope 1997;107(8):1043-1047.
91. Betchen SA, Walsh J, Post KD. Long-term hearing preservation after surgery for vestibular schwannoma. J Neurosurg 2005;102(1):6-9.
92. Brackmann DE, House 3rd JR, Hitselberger WE. Technical modifications to the middle fossa craniotomy approach in removal of acoustic neuromas. Am J Otol 1994;15(5):614-619.
93. Brackmann DE, Fayad JN, Slattery 3rd WH, et al. Early proactive management of vestibular schwannomas in neurofibromatosis type 2. Neurosurgery 2001;49(2):274-280.
94. Chee GH, Nedzelski JM, Rowed D. Acoustic neuroma surgery: the results of long-term hearing preservation. Otol Neurotol 2003;24(4):672-676.
95. Colletti V, Fiorino F. Middle fossa versus retrosigmoid-transmeatal approach in vestibular schwannoma surgery: a prospective study. Otol Neurotol 2003;24(6):927-934.
96. Dornhoffer JL, Helms J, Hoehmann DH. Hearing preservation in acoustic tumor surgery: results and prognostic factors. Laryngoscope 1995;105(2):184-187.
97. Irving RM, Jackler RK, Pitts LH. Hearing preservation in patients undergoing vestibular schwannoma surgery: comparison of middle fossa and retrosigmoid approaches. J Neurosurg 1998;88(5):840-845.
98. Harner SG, Fabry DA, Beatty CW. Audiometric findings in patients with acoustic neuroma. Am J Otol 2000;21(3):405-411.
99. Ginzkey C, Scheich M, Harnisch W, et al. Outcome on hearing and facial nerve function in microsurgical treatment of small vestibular schwannoma via the middle cranial fossa approach. Eur Arch Otorhinolaryngol 2013;270(4):1209-1216.
100. Kutz Jr JW, Scoresby T, Isaacson B, et al. Hearing preservation using the middle fossa approach for the treatment of vestibular schwannoma. Neurosurgery 2012;70(2):334-340.
101. Lin VY, Stewart C, Grebenyuk J, et al. Unilateral acoustic neuromas: long-term hearing results in patients managed with fractionated stereotactic radiotherapy, hearing preservation surgery, and expectantly. Laryngoscope 2005;115(2):292-296.
102. Maw AR, Coakham HB, Ayoub O, Butler SR. Hearing preservation and facial nerve function in vestibular schwannoma surgery. Clin Otolaryngol Allied Sci 2003;28(3):252-256.
103. Rigby PL, Shah SB, Jackler RK, Chung JH, Cooke DD. Acoustic neuroma surgery: outcome analysis of patient-perceived disability. Am J Otol 1997;18(4):427-435.
104. Samii M, Matthies C. Hearing preservation in acoustic tumour surgery. Adv Tech Stand Neurosurg 1995;22:343-373.
105. Samii M, Gerganov V, Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg 2006;105(4):527-535.
106. Sanna M, Khrais T, Russo A, Piccirillo E, Augurio A. Hearing preservation surgery in vestibular schwannoma: the hidden truth. Ann Otol Rhinol Laryngol 2004;113(2):156-163.
107. Slattery 3rd WH, Brackmann DE, Hitselberger W. Middle fossa approach for hearing preservation with acoustic neuromas. Am J Otol Sep 1997;18(5):596-601.
108. Slattery 3rd WH, Brackmann DE, Hitselberger W. Hearing preservation in neurofibromatosis type 2. Am J Otol 1998;19(5):638-643.
109. Weber PC, Gantz BJ. Results and complications from acoustic neuroma excision via middle cranial fossa approach. Am J Otol 1996;17(4):669-675.
110. Wiet RJ, Mamikoglu B, Odom L, Hoistad DL. Long-term results of the first 500 cases of acoustic neuroma surgery. Otolaryngol Head Neck Surg 2001;124(6):645-651.
111. Glasscock 3rd ME, Hart MJ, Vrabec JT. Management of bilateral acoustic neuroma. Otolaryngol Clin North Am 1992;25(2):449-469.
112. Samii M, Matthies C, Tatagiba M. Management of vestibular schwannomas (acoustic neuromas): auditory and facial nerve function after resection of 120 vestibular schwannomas in patients with neurofibromatosis 2. Neurosurgery 1997;40(4):696-705.
113. Slattery 3rd WH, Fisher LM, Hitselberger W, Friedman RA, Brackmann DE. Hearing preservation surgery for neurofibromatosis type 2-related vestibular schwannoma in pediatric patients. J Neurosurg 2007;106(4 suppl):255-260.
114. Friedman RA, Goddard JC, Wilkinson EP, et al. Hearing preservation with the middle cranial fossa approach for neurofibromatosis type 2. Otol Neurotol 2011;32(9):1530-1537.
115. Tysome JR, Macfarlane R, Durie-Gair J, et al. Surgical management of vestibular schwannomas and hearing rehabilitation in neurofibromatosis type 2. Otol Neurotol 2012;33(3):466-472.
116. Buchman CA, Chen DA, Flannagan P, Wilberger JE, Maroon JC. The learning curve for acoustic tumor surgery. Laryngoscope 1996;106(11):1406-1411.
117. Fusco MR, Fisher WS, McGrew BM, Walters BC. Current practices in vestibular schwannoma management: a survey of American and Canadian neurosurgeons. Clin Neurol Neurosurg 2014;127:143-148.
118. Goodden JR, Tranter R, Hardwidge C. Setting the standard—UK neurosurgical acoustic neuroma practice. Ann R Coll Surg Engl 2006;88(5):486-489.
119. Tonn JC, Schlake HP, Goldbrunner R, Milewski C, Helms J, Roosen K. Acoustic neuroma surgery as an interdisciplinary approach: a neurosurgical series of 508 patients. J Neurol Neurosurg Psychiatry 2000;69(2):161-166.
120. British Association of Otorhinolaryngologists – Head and Neck Surgeons Clinical Practice Advisory Group. Clinical Effectiveness Guidelines Acoustic Neuroma (Vestibular Schwannoma) 2002. BAO-HNS document 5.
121. Anaizi AN, Gantwerker EA, Pensak ML, Theodosopoulos PV. Facial nerve preservation surgery for koos grade 3 and 4 vestibular schwannomas. Neurosurgery 2014;75(6):671-675.
122. Pollock BE, Lunsford LD, Flickinger JC, Clyde BL, Kondziolka D. Vestibular schwannoma management. Part I. Failed microsurgery and the role of delayed stereotactic radiosurgery. J Neurosurg 1998;89(6):944-948.
123. Pollock BE, Link MJ. Vestibular schwannoma radiosurgery after previous surgical resection or stereotactic radiosurgery. Prog Neurol Surg 2008;21:163-168.
124. Unger F, Walch C, Papaefthymiou G, Feichtinger K, Trummer M, Pendl G. Radiosurgery of residual and recurrent vestibular schwannomas. Acta Neurochir (Wien) 2002;144(7):671-676.
125. Brokinkel B, Sauerland C, Holling M, et al. Gamma Knife radiosurgery following subtotal resection of vestibular schwannoma. J Clin Neurosci 2014;21(12):2077-2082.
126. Fuentes S, Arkha Y, Pech-Gourg G, Grisoli F, Dufour H, Regis J. Management of large vestibular schwannomas by combined surgical resection and gamma knife radiosurgery. Prog Neurol Surg 2008;21:79-82.
127. Iwai Y, Yamanaka K, Ishiguro T. Surgery combined with radiosurgery of large acoustic neuromas. Surg Neurol 2003;59(4):283-289.
128. Pan HC, Sheehan J, Sheu ML, Chiu WT, Yang DY. Intracapsular decompression or radical resection followed by Gamma Knife surgery for patients harboring a large vestibular schwannoma. J Neurosurg 2012;117(suppl):69-77.
129. Park CK, Jung HW, Kim JE, Son YJ, Paek SH, Kim DG. Therapeutic strategy for large vestibular schwannomas. J Neurooncol 2006;77(2):167-171.
130. Porter RG, LaRouere MJ, Kartush JM, Bojrab DI, Pieper DR. Improved facial nerve outcomes using an evolving treatment method for large acoustic neuromas. Otol Neurotol 2013;34(2):304-310.
131. van de Langenberg R, Hanssens PE, van Overbeeke JJ, et al. Management of large vestibular schwannoma. Part I. Planned subtotal resection followed by Gamma Knife surgery: radiological and clinical aspects. J Neurosurg 2011;115(5):875-884.
132. Virk JS, Tripathi S, Randhawa PS, Kwasa EA, Mendoza ND, Harcourt J. Tumour resection volumes and facial nerve outcomes for vestibular schwannomas. Indian J Otolaryngol Head Neck Surg 2014;66(2):191-195.
133. Pollock BE, Driscoll CL, Foote RL, et al. Patient outcomes after vestibular schwannoma management: a prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery 2006;59(1):77-85.
134. Myrseth E, Moller P, Pedersen PH, Lund-Johansen M. Vestibular schwannoma: surgery or gamma knife radiosurgery? A prospective, nonrandomized study. Neurosurgery 2009;64(4):654-661.
135. Stavas MJ, Carlson ML, Attia A, et al. Does radiation dose to the vestibule predict change in balance function and patient perceived dizziness following stereotactic radiotherapy for vestibular schwannoma? Am J Otolaryngol 2014;35(5):565-571.
136. Varughese JK, Wentzel-Larsen T, Pedersen PH, Mahesparan R, Lund-Johansen M. Gamma knife treatment of growing vestibular schwannoma in Norway: a prospective study. Int J Radiat Oncol Biol Phys 2012;84(2):e161-166.
137. Humphriss RL, Baguley DM, Axon PR, Moffat DA. Change in hearing handicap after translabyrinthine vestibular schwannoma excision. Otol Neurotol 2004;25(3):371-378.
138. Kane NM, Kazanas S, Maw AR, et al. Functional outcome in patients after excision of extracanalicular acoustic neuromas using the suboccipital approach. Ann R Coll Surg Engl 1995;77(3):210-216.
139. Driscoll CL, Lynn SG, Harner SG, Beatty CW, Atkinson EJ. Preoperative identification of patients at risk of developing persistent dysequilibrium after acoustic neuroma removal. Am J Otol 1998;19(4):491-495.
140. Karpinos M, Teh BS, Zeck O, et al. Treatment of acoustic neuroma: stereotactic radiosurgery vs. microsurgery. Int J Radiat Oncol Biol Phys 2002;54(5):1410-1421.
141. Timmer FC, van Haren AE, Mulder JJ, et al. Quality of life after gamma knife radiosurgery treatment in patients with a vestibular schwannoma: the patient’s perspective. Eur Arch Otorhinolaryngol 2010;267(6):867-873.
142. Feigl GC, Schebesch KM, Rochon J, et al. Analysis of risk factors influencing the development of severe dizziness in patients with vestibular schwannomas in the immediate postoperative phase. Clin Neurol Neurosurg 2011;113(1):52-56.
143. Wagner JN, Glaser M, Wowra B, et al. Vestibular function and quality of life in vestibular schwannoma: does size matter? Front Neurol 2011;2:55.
144. Carlson ML, Tveiten OV, Driscoll CL, et al. Long-term dizziness handicap in patients with vestibular schwannoma: a multicenter cross-sectional study. Otolaryngol Head Neck Surg 2014;151(6):1028-1037.
145. Al-Shudifat AR, Kahlon B, Hoglund P, Soliman AY, Lindskog K, Siesjo P. Age, gender and tumour size predict work capacity after surgical treatment of vestibular schwannomas. J Neurol Neurosurg Psychiatry 2014;85(1):106-111.
146. Robinett ZN, Walz PC, Miles-Markley B, Moberly AC, Welling DB. Comparison of long-term quality-of-life outcomes in vestibular schwannoma patients. Otolaryngol Head Neck Surg 2014;150(6):1024-1032.
147. Badakhshi H, Muellner S, Wiener E, Budach V. Image-guided stereotactic radiotherapy for patients with vestibular schwannoma. A clinical study. Strahlenther Onkol 2014;190(6):533-537.
148. Karkas A, Lamblin E, Meyer M, Gay E, Ternier J, Schmerber S. Trigeminal nerve deficit in large and compressive acoustic neuromas and its correlation with MRI findings. Otolaryngol Head Neck Surg 2014;151(4):675-680.
149. Squire SE, Chan MD, Furr RM, et al. Gamma knife radiosurgery in the treatment of tumor-related facial pain. Stereotact Funct Neurosurg 2012;90(3):145-150.
150. Koh ES, Millar BA, Menard C, et al. Fractionated stereotactic radiotherapy for acoustic neuroma: single-institution experience at The Princess Margaret Hospital. Cancer 2007;109(6):1203-1210.
151. Prasad D, Steiner M, Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg 2000;92(5):745-759.
152. Barker 2nd FG, Jannetta PJ, Babu RP, Pomonis S, Bissonette DJ, Jho HD. Long-term outcome after operation for trigeminal neuralgia in patients with posterior fossa tumors. J Neurosurg 1996;84(5):818-825.
153. Puca A, Meglio M, Vari R, Tamburrini G, Tancredi A. Evaluation of fifth nerve dysfunction in 136 patients with middle and posterior cranial fossae tumors. Eur Neurol 1995;35(1):33-37.
154. Samii M, Matthies C. Acoustic neurinomas associated with vascular compression syndromes. Acta Neurochir (Wien) 1995;134(3-4):148-154.
155. Lee CC, Wu HM, Chung WY, Chen CJ, Pan DH, Hsu SP. Microsurgery for vestibular schwannoma after Gamma Knife surgery: challenges and treatment strategies. J Neurosurg 2014;121(suppl):150-159.
156. Hong B, Krauss JK, Bremer M, Karstens JH, Heissler HE, Nakamura M. Vestibular schwannoma microsurgery for recurrent tumors after radiation therapy or previous surgical resection. Otol Neurotol 2014;35(1):171-181.
157. Gerganov VM, Giordano M, Samii A, Samii M. Surgical treatment of patients with vestibular schwannomas after failed previous radiosurgery. J Neurosurg 2012;116(4):713-720.
158. Friedman RA, Berliner KI, Bassim M, et al. A paradigm shift in salvage surgery for radiated vestibular schwannoma. Otol Neurotol 2011;32(8):1322-1328.
159. Lee CC, Yen YS, Pan DH, et al. Delayed microsurgery for vestibular schwannoma after gamma knife radiosurgery. J Neurooncol 2010;98(2):203-212.
160. Liscak R, Vladyka V, Urgosik D, Simonova G, Vymazal J. Repeated treatment of vestibular schwannomas after gamma knife radiosurgery. Acta Neurochir (Wien) 2009;151(4):317-324.
161. Shuto T, Inomori S, Matsunaga S, Fujino H. Microsurgery for vestibular schwannoma after gamma knife radiosurgery. Acta Neurochir (Wien) 2008;150(3):229-234.
162. Pollock BE. Management of vestibular schwannomas that enlarge after stereotactic radiosurgery: treatment recommendations based on a 15 year experience. Neurosurgery 2006;58(2):241-248.
163. Friedman RA, Brackmann DE, Hitselberger WE, Schwartz MS, Iqbal Z, Berliner KI. Surgical salvage after failed irradiation for vestibular schwannoma. Laryngoscope 2005;115(10):1827-1832.
164. Pollock BE, Lunsford LD, Kondziolka D, et al. Vestibular schwannoma management. Part II. Failed radiosurgery and the role of delayed microsurgery. J Neurosurg 1998;89(6):949-955.
© Congress of Neurological Surgeons
Source: Neurosurgery, February 2018