Guidelines For Pediatric Myelomeningocele
2. The Incidence of Shunt-Dependent Hydrocephalus in Infants with Myelomeningocele After Prenatal versus Postnatal Repair
Download pdf Neurosurgery, 2019
Sponsored by: Congress of Neurological Surgeons (CNS) and the Section on Pediatric Neurosurgery
Endorsed by: The Congress of Neurological Surgeons (CNS), American Association of Neurological Surgeons (AANS), and Spina Bifida Association (SBA)
Mandeep S. Tamber, MD, PhD1, Ann Marie Flannery, MD2, Catherine McClung-Smith, MD3, Nadege Assassi4, David F. Bauer, MD5, Alexandra D. Beier, DO6, Jeffrey P. Blount, MD7, Susan R. Durham, MD MS8, Paul Klimo Jr., MD9, Dimitrios C. Nikas, MD10, Patricia Rehring, MPH11, Rachana Tyagi, MD12, Catherine A. Mazzola, MD13
- Division of Pediatric Neurosurgery, British Columbia Children’s Hospital, University of British Columbia, Vancouver, British Columbia, Canada
- Kids Specialty Center, Women’s & Children’s Hospital, Lafayette, Louisiana
- Department of Neurological Surgery, Palmetto Health USC Medical Group, Columbia, South Carolina
- Department of Surgery, Division of Neurosurgery, Robert Wood Johnson Medical School, New Brunswick, New Jersey
- Department of Surgery, Division of Neurosurgery, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire
- Division of Pediatric Neurosurgery, University of Florida Health Jacksonville, Jacksonville, Florida
- Division of Pediatric Neurosurgery, Department of Neurosurgery, University of Alabama at Birmingham; Children’s of Alabama, Birmingham, Alabama
- The University of Vermont Medical Center, Burlington, Vermont
- Semmes-Murphey; Department of Neurosurgery, University of Tennessee Health Science Center; Le Bonheur Children’s Hospital, Memphis, Tennessee
- Division of Pediatric Neurosurgery, Advocate Children's Hospital, Oak Lawn, Illinois
- Congress of Neurological Surgeons, Schaumburg, Illinois
- Department of Neurosurgery, Mercer University Medical School, Macon, Georgia
- Goryeb Children’s Hospital, Morristown, New Jersey; Rutgers Department of Neurological Surgery, Newark, New Jersey
Correspondence:
Mandeep S. Tamber, MD, PhD
Division of Pediatric Neurosurgery
British Columbia Children’s Hospital
Room K3-159, 4480 Oak Street
Vancouver, BC V6H 3V4
mandeep.tamber@cw.bc.ca
Abbreviations:
COI- conflict of interest
CSF - Cerebrospinal fluid
MOMS - Management of Myelomeningocele Study
MM – myelomeningocele
SB – spina bifida
ABSTRACT
Background: Myelomeningocele (MM) is a condition that is responsible for considerable morbidity in the pediatric population. A significant proportion of the morbidity related to MM is attributable to hydrocephalus and the surgical management thereof. Postnatal repair remains the most common form of treatment, however, increased rates of prenatal diagnosis, advances in fetal surgery and a hypothesis that neural injury continues in utero until the MM defect is repaired have led to the development and evaluation of prenatal surgery as a means to improve outcomes in afflicted infants.
Objective: The objective of this guideline is to systematically evaluate the literature to determine whether there is a difference in the proportion of patients who develop shunt-dependent hydrocephalus in infants who underwent prenatal MM repair compared to infants who had postnatal repair.
Methods: The Guidelines Task Force developed search terms and strategies used to search PubMed and Embase for relevant literature published between 1966 and September 2016. Strict inclusion/exclusion criteria were used to screen abstracts and to develop a list of relevant articles for full-text review. Full-text articles were then reviewed, and when appropriate, included as evidence.
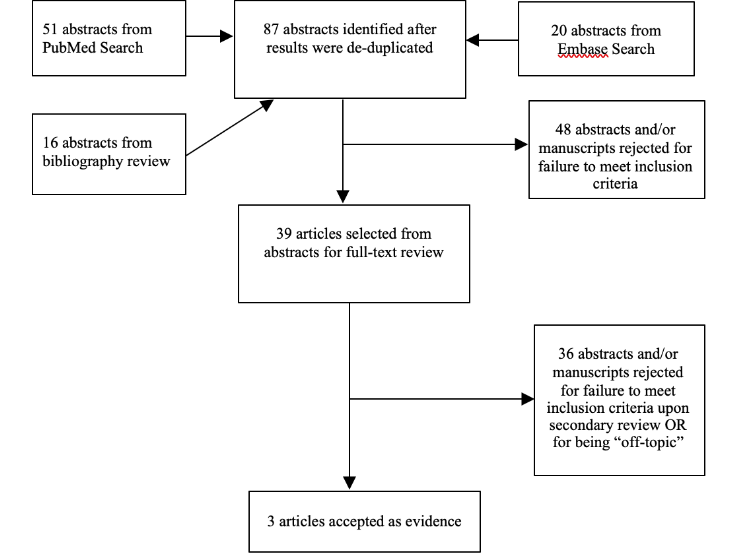
Results: A total of 87 abstracts were identified and reviewed by 3 independent reviewers. Thirty-nine full-text articles were selected for analysis. Three studies met selection criteria and were included in the evidence table.
Conclusions: Class I evidence from 1 study and Class III evidence from 2 studies suggest that, in comparison to postnatal repair, prenatal surgery for MM reduces the risk of developing shunt-dependent hydrocephalus. Therefore, prenatal repair of MM is recommended for those fetuses who meet specific criteria for prenatal surgery to reduce the risk of developing shunt-dependent hydrocephalus (Level I). Differences between prenatal and postnatal repair with respect to the requirement for permanent cerebrospinal fluid diversion should be considered alongside other relevant maternal and fetal factors when deciding upon a preferred method of MM closure.
Keywords: Fetal, hydrocephalus, in utero closure, myelomeningocele, postnatal, shunt, spina bifida
RECOMMENDATIONS
PICO Question: Is there a difference in the proportion of patients who develop shunt-dependent hydrocephalus between fetuses who underwent prenatal myelomeningocele closure compared to infants who underwent postnatal myelomeningocele repair?
Target Population: Infants with myelomeningocele who meet eligibility criteria as fetuses for prenatal myelomeningocele repair.
Recommendation(s): Prenatal repair of myelomeningocele is recommended for those fetuses who meet maternal and fetal MOMS specified criteria for prenatal surgery to reduce the risk of developing shunt-dependent hydrocephalus (Level I). Differences between prenatal and postnatal repair with respect to the requirement for permanent cerebrospinal fluid diversion should be considered alongside other relevant maternal and fetal factors when deciding upon a preferred method of myelomeningocele closure.
INTRODUCTION
Rationale
The approximate incidence of myelomeningocele (MM) in the United States is 3.4 per 10000 live births.1 Live-born infants with MM have an approximately 10% risk of mortality, and survivors face significant morbidity.2, 3
Conventional treatment of MM has been to perform a postnatal repair within 48 hours after birth. After repair of the defect, neurosurgeons must remain vigilant about recognizing and treating hydrocephalus, which is a frequent comorbidity in MM patients.
In limited studies where fetuses with MM were serially examined with ultrasound, progressive reduction in lower extremity movement has been observed.4, 5 It has been hypothesized that ongoing irritation of the neural placode by amniotic fluid may damage already compromised neural tissue, resulting in progressive neurological decline. Moreover, continual cerebrospinal fluid (CSF) leak from the open defect may worsen hindbrain herniation and contribute to the development of hydrocephalus.6 These observations have led clinicians to explore whether in utero repair of the MM defect may result in improved patient outcomes.
Early observations regarding the effect of prenatal MM repair on outcomes were published in the 1990’s,7-9 and demonstrated that some fetuses who received in utero repair had improved functional outcomes and a lower requirement for shunt placement after birth. Outcomes in subsequent larger cohorts of infants who were treated with prenatal surgery as fetuses, when compared to historical controls treated with postnatal repair, continued to demonstrate evidence of improved patient functional outcomes and a lower incidence of shunt-dependent hydrocephalus.10, 11
Prenatal repair of MM involves serious and permanent risks for mother and fetus. Fetal demise, preterm labor, premature rupture of membranes and maternal infection are of particular concern. The Management of Myelomeningocele Study (MOMS) trial was the first and only randomized comparison of the safety and efficacy of prenatal MM repair with respect to conventional postnatal surgery.12
No evidence-based guidelines regarding the role of prenatal surgery in the management of MM were published prior to this guideline. Given the potentially important risks and benefits to the infant and mother, the authors felt that it was important to evaluate the literature in a systematic way in an effort to guide clinicians in the management of this challenging disorder.
In this guideline, the authors specifically address whether the incidence of shunt-dependent hydrocephalus differs between fetuses with MM who underwent prenatal vs infants with MM who underwent postnatal repair.
OBJECTIVES
The objective of this guideline was to comprehensively search the current literature, evaluate the evidence and make appropriate recommendations for the management of patients with MM, with specific attention focused on the question of whether prenatal repair of MM results in a smaller proportion of infants who develop shunt-dependent hydrocephalus compared to those who undergo conventional postnatal repair.
METHODS
Writing Group and Question Establishment
The Guidelines Task Force initiated a systematic review of the literature and evidence-based guideline relevant to the diagnosis and treatment of patients with MM. Through objective evaluation of the evidence and transparency in the process of making recommendations, this evidence-based clinical practice guideline was developed for the diagnosis and treatment of patients with MM. These guidelines are developed for educational purposes to assist practitioners in their clinical decision-making processes. Additional information about the methods utilized in this systematic review is provided in the introduction and methodology chapter.
Literature Search
The task force members collaborated with a medical librarian to search the PubMed and Embase databases for the period from 1966 to September 2016 using the search strategies provided in Appendix I. The literature search yielded 71 abstracts. To supplement the results of the electronic search, an additional 16 abstracts/articles were identified after manually screening the bibliographies of all retrieved publications. The task force selected 39 full-text articles for review. Of these, 36 were rejected for not meeting inclusion criteria or for being off-topic. Three were selected for systematic review.
Study Selection and Eligibility Criteria
A total of 39 citations were manually reviewed by the team using specific inclusion and exclusion criteria as outlined below. Three independent reviewers evaluated and abstracted full-text data for each article, and the sets of data were compared for agreement. Inconsistencies were re-reviewed, and disagreements were resolved by consensus. The citations were reviewed by the team using the following inclusion and exclusion criteria:
- At least 80% of patients had to be patients with MM and <18 years of age.
- Studies that enrolled >20% of patients with other forms of spina bifida (SB) were excluded.
- Studies that combined the results of patients with other forms of SB were excluded if the study enrolled less than 80% of target patient population.
- Studies that enrolled mixed patient populations were included only if they reported separate results for the target population. The results of the target population were the only results considered as evidence to support our recommendations.
- The study was a full article report of a clinical study.
- The study was not a meeting abstract, editorial, letter, or a commentary.
- Prospective case series had to report baseline values, if applicable.
- Case series studies with non-consecutive enrollment of patients were excluded.
- Studies had to have appeared in a peer-reviewed publication or a registry report.
- Studies had to enroll at least 10 patients for each distinct outcome measured. If it was a comparative study, a minimum enrollment of 5 patients per treatment arm for each outcome was necessary.
- The study involved humans.
- The study was published between January 1966 and September 2016.
- The study presented results quantitatively.
- The study did not involve “in vitro”, “biomechanical” or results performed on cadavers.
- The study was published in English.
- Papers reporting results of systematic reviews, meta-analyses, or guidelines developed by others were excluded.
- Authors specifically excluded follow-up studies in which a cohort of patients from an initial study were followed in time and separately reported upon in a subsequent publication. This prevented the same patients from being included multiple times in this review.
To reduce bias, these criteria were specified before conducting the literature searches. For the purposes of this evidence review, articles that did not meet the selection criteria are not evidence and not considered as potential evidence to support the clinical recommendations. These same criteria were also applied to the 16 additional articles identified during the bibliographic review.
The authors did not include systematic reviews, guidelines, or meta-analyses conducted by others. These documents were developed using different inclusion criteria than those specified in this guideline. Therefore, they may include studies that do not meet the inclusion criteria specified above. These documents were recalled if their abstract suggested that they might address one of the recommendations, and their bibliographies were searched for additional studies. Of the 39 articles selected for full text review, 36 were rejected for not meeting inclusion criteria or for being off-topic. There were 3 studies that met inclusion criteria.9, 13, 14 See PRISMA Article Flow Chart in Appendix II.
Data Collection Process
The abstracts that met the selection criteria mentioned above were retrieved in full-text form. Each article’s adherence to the selection criteria was assessed. To determine how the data could be classified, the information in the full-text articles was then evaluated to determine whether they were providing results of therapy or were more centered on diagnostic or prognostic information. Agreement on these assessments and on the salient points regarding the type of study design and objectives, and the conclusions and data classification was then reached by exchanging drafts and comments by e-mail. The information was then used for construction of the evidence tables.
Assessment for Risk of Bias
The methodological quality of RCTs and the risk of bias were assessed using the following six criteria:
- Sequence generation (Was the allocation sequence adequately generated?)
- Allocation concealment (Was allocation adequately concealed such that it could not be foretold?)
- Blinding (Were participants, treatment providers and/or outcome assessors blinded to the treatment allocations?)
- Incomplete reporting of data (Were incomplete outcome data adequately addressed?)
- Selective reporting of outcomes (Were all the outcomes specified reported?)
- Other potential threats to validity (Was the RCT free of other issues that could put it at a high risk of bias?)
In the case of non-randomized observational evidence, potential threats to the validity of the data were assessed by examining for:
- bias due to selective case choice for study and selective result reporting,
- bias due lack or loss of information over time,
- the biases of the interpreting investigator in regard to the study,
- publication bias regarding positive studies or positive cases,
- misclassification,
- survivorship bias,
- publication bias,
- recognition that in data collected in a retrospective or prospective manner correlation does not imply causation,
- election bias,
- attrition bias,
- bias of change in methods over time,
- ascertainment bias,
Rating Quality of Evidence
The quality of evidence was rated using an evidence hierarchy for therapeutic studies. Demonstrating the highest degree of clinical certainty, Class I evidence is used to support recommendations of the strongest type, defined as Level I recommendations. Level II recommendations reflect a moderate degree of clinical certainty and are supported by Class II evidence. Level III recommendations denote clinical uncertainty supported by Class III evidence. This hierarchy is shown in Appendix III. Additional information regarding the hierarchy classification of evidence is found here on the CNS Guidelines methodology page.
Revision Plans
In accordance with the Institute of Medicine’s standards for developing clinical practice guidelines the task force will monitor related publications following the release of this document and will revise the entire document and/or specific sections “if new evidence shows that a recommended intervention causes previously unknown substantial harm; that a new intervention is significantly superior to a previously recommended intervention from an efficacy or harms perspective; or that a recommendation can be applied to new populations.”15 In addition, the task force will confirm within 5 years from the date of publication that the content reflects current clinical practice and the available technologies regarding the clinical issue of the incidence of shunt-dependent hydrocephalus in infants with MM after prenatal vs. postnatal repair.
RESULTS
Study Selection and Characteristics
The 3 studies that inform this recommendation are of varying methodological design and quality. There were 2 prospective13, 14 and 1 retrospective9 comparative studies which directly compared various outcomes, including the need for CSF shunt placement, between fetuses treated with prenatal MM repair and those infants who received traditional postnatal surgery. The studies that were included in this guideline will be discussed in detail in the following section (see Appendix IV).
Several studies that were reviewed in full text were subsequently excluded because they did not meet the criteria for inclusion (see Appendix II). Nevertheless, these studies do merit some discussion, as they provide supportive information that is relevant to the topic of this guideline. Because they were case series with non-consecutive enrolment, 2 studies that reported the outcomes of patients who had prenatal surgery in comparison to historically assembled cohorts of infants who had postnatal MM repair10, 11 were excluded from the evidence table. For the same reason, several retrospective, single arm cases series providing evidence regarding the proportion of patients who required CSF shunt placement following either postnatal16-21 or prenatal22, 23 MM repair were also excluded.
Given the limited number of fetuses who have been treated with in utero MM repair, the evidence review task force paid attention to avoid including multiple studies in the evidentiary tables that appeared to be reporting results on the same cohort of patients. In light of this concern, readers will notice that the MOMS12 was not included in the evidence table for this recommendation, because the full data from this randomized trial was eventually reported in a 2015 publication that was included in the evidence table13. Although none of these studies met criteria for inclusion into the evidence table, it also appears that multiple publications detailing the outcomes of the same group of patients treated with prenatal MM repair at the Children’s Hospital of Philadelphia between 1998 and 2002 are present in the literature.22, 24, 25
Results of Individual Studies, Discussion of Study Limitations and Risk of Bias
The MOMS Study was a multicenter randomized controlled trial comparing the safety and efficacy of prenatal vs. postnatal MM repair.12 Safety and efficacy were judged using a composite primary outcome consisting of fetal or neonatal death or the placement of a CSF shunt at 12 months. The trial was stopped early for efficacy after recruitment of 183 of a planned 200 participants because it demonstrated that fetuses who underwent prenatal surgery had a lower CSF shunt placement rate (40%) than those who had standard postnatal repair (82%) (p<0.001). In 2015, the MOMS investigators presented the updated one-year outcomes for the 183 patients who completed this randomized controlled trial.13 The updated data demonstrated that the actual proportion of patients who needed CSF shunt placement at one year was 44% in the prenatal surgery arm and 84% in the postnatal surgery arm (p<0.0001). Each component of the primary outcome, including the need for shunt placement, was independently adjudicated by an external group of blinded pediatric neurosurgeons. No difference was observed between groups with respect to the safety component of the primary outcome; there was one fetal and one neonatal death in the prenatal surgery group and 2 neonatal deaths in the postnatal surgery group.13 Of note, the safety component of the composite primary outcome addressed some of the risks to the fetus (death), but not all (eg, non-fatal sequelae of preterm delivery). In addition, risks specific to the mother were not captured by the chosen primary outcome.
MOMS was a well-designed, conducted and reported randomized control trial which was deemed to supply Class I evidence regarding this recommendation. However, several caveats should be recognized when interpreting the results of this trial. Clinicians should be aware of the limited generalizability (external validity) of the results of this trial. Fetal surgery was, and continues to be, offered at highly specialized institutions that have refined their experience with the required techniques over many years. Clinicians should also recognize that even though fetal MM repair is an evolving technique with a broadening inclusion criteria for patients, the patients included in MOMS were highly selected for on the basis of very strict trial inclusion and exclusion criteria.12 Mothers 18 years of age and older carrying a singleton pregnancy were considered eligible for MOMS. For inclusion into the trial, fetuses were required to have a normal karyotype, a gestational age of 19-25.9 weeks at randomization, and a MM defect with an upper level between T1 and S1 with evidence of hindbrain herniation. Major exclusion criteria were a severe kyphotic deformity related to MM, a fetal anomaly unrelated to MM, as well as a risk of preterm birth, placental abruption or any other contraindication to surgery (see Appendix V).
A comment should also be made about the decision to place a CSF shunt (the outcome measure). The MOMS investigators appreciated the often subjective nature of the decision to place a CSF shunt in a child, and, as a result, developed an a priori set of criteria for shunt placement that was used by a panel of pediatric neurosurgeons to adjudicate each study event. When the complete data was analyzed, it was observed that a significant proportion of patients enrolled in MOMS met the criteria for shunt placement but did not undergo the procedure.13 This observation was likely suggestive of an evolution of indications for shunt placement, reflecting an overall trend amongst pediatric neurosurgeons to rely much more heavily on overt symptoms and signs of hydrocephalus rather than progressive ventriculomegaly alone as a criteria for shunt placement.
The lack of methodological rigor of 2 other comparative studies led to their demotion from Class II to Class III evidence.9, 14 Although both studies demonstrated that the proportion of infants requiring permanent CSF diversion was significantly lower in those infants who had prenatal MM repair compared to those who had postnatal repair, important issues with respect to study design and reporting limit the impact of this data on the eventual recommendation.
The study by Zamlynski et al14 was a prospective nonrandomized comparison of outcomes. Pregnant mothers of fetuses diagnosed with MM who were candidates for either prenatal or postnatal repair were allowed to choose which operation they preferred (selection bias). Many patients in the postnatal surgery group underwent repairs that were not compliant with study criteria and were excluded from the analysis. A significant number of patients were lost to follow-up, leading to concerns regarding ascertainment bias. Although not a methodological weakness per se, it should be noted that this study used different inclusion and exclusion criteria for determining candidacy for in utero surgery (notably the inclusion of low sacral MMs and the exclusion of thoracic lesions) than those established by the MOMS investigators. It should also be noted that this study reported that 28% of infants that met criteria as fetuses who underwent prenatal MM closure required CSF diversion, a figure much lower than that reported by the MOMS investigators, and perhaps the result of the biases outlined above.
Bruner et al9 also reported a nonrandomized study that suffers from selection bias because participants selected their intervention (prenatal or postnatal repair). Their technique of prenatal repair also changed during the course of the study. Because of its non-randomized design, the prenatal and postnatal surgery groups had significant imbalances in baseline characteristics, with patients in the prenatal repair group more likely to be older and previously pregnant/given birth than patients in the postnatal surgery group. Specific criteria for shunt placement were not imposed. Whereas 59% of prenatal repair infants required shunt insertion, 91% of patients in the postnatal group had a CSF shunt placed. The latter figure is higher than that which would be expected based on the MOMS data and other earlier series of postnatal MM repair (see below).
DISCUSSION
Biases inherent to small cases series limit the quality of the data they can provide. Nevertheless, the foundation of the current recommendation can be somewhat strengthened if these case series provide data which is congruent with more methodologically rigorous studies. Several case series, with or without the use of historical controls, were identified during the process of initial abstract screening. Although they did not meet the criteria for inclusion into the evidence table, they do provide some corroborating information relevant to this recommendation.
Two studies employed historical controls treated with postnatal repair as a benchmark to evaluate the effect of prenatal MM closure on the requirement for shunt placement.10, 11 Both of these studies provided supportive evidence in favor of the magnitude and direction of the effect observed in the subsequently completed MOMS trial.
Several retrospective, non-comparative cases series provided evidence regarding the proportion of patients who required CSF shunt placement following either postnatal16-21 or prenatal22, 23 MM repair. The results of these small studies report shunt placement rates generally around 80% in postnatal repair infants and 50% in prenatal repair patients, largely in keeping with the eventual results of the MOMS trial.12, 13 As emphasized by the MOMS investigators, the lack of uniform criteria for the initiation of permanent CSF diversion therapy can significantly influence the likelihood of this outcome. To illustrate this point further, by using rigorous and “stringent” criteria for the placement of a CSF shunt, clinicians at Great Ormond Street Hospital were able to demonstrate that the shunt insertion rate in a contemporary cohort who underwent postnatal MM repair could be lower than previously reported and comparable to that following in utero repair.18
There appears to be evidence that prenatal MM repair is effective in reducing the proportion of infants who require CSF shunt placement when compared to infants who are treated using conventional postnatal repair. The most robust data comes from a single randomized controlled trial, although there is other evidence in support of the direction and magnitude of the observed effect. Based on a thorough systematic review of the literature, this guideline can provide a Level I recommendation that prenatal repair of MM is recommended for those fetuses that meet maternal and fetal MOMS inclusion criteria to reduce the risk of developing shunt-dependent hydrocephalus. Differences between prenatal and postnatal repair with respect to the requirement for permanent CSF diversion should be considered alongside other relevant maternal and fetal factors, such as the likelihood of preterm delivery, when deciding upon a preferred method of MM closure.
Despite the strength if the recommendation, readers of this guideline should be cognizant of important caveats regarding generalizability. This recommendation is based upon data from a very small number of patients because of the highly restrictive criteria that are used to judge whether an individual is appropriate for prenatal intervention. As a corollary, the recommendation is only applicable to patients who are considered potential candidates for prenatal MM repair based upon those same criteria. In addition, there is uncertainty regarding whether the documented effect of prenatal MM repair on the incidence of shunt dependent hydrocephalus can be replicated outside of the highly experienced centers involved in MOMS. This issue further limits the potential impact of this recommendation to the general MM population.
Future Research
Fetal surgery for MM is a potentially major advance in pediatric neurosurgery. As with most surgical interventions, the technique continues to evolve. Minimally invasive fetoscopic techniques have been developed and small outcome series have been published. Study of fetoscopic MM repair should be pursued with similar rigor as that which was established by MOMS.
As noted above, given the large numbers of infants born with MM each year, the limited generalizability of fetal repair remains a significant challenge with respect to the broad applicability of this recommendation. As experience with the technique matures, all reasonable efforts should be made not only to increase the number of centers capable of offering this intervention, but also to liberalize the eligibility criteria for prenatal surgery. This is essential given the narrow inclusion criteria of the original MOMS trial. Given the risk associated with prenatal intervention, it is also imperative to precisely define the subset of patients that are most likely to benefit from prenatal surgery. To that end, the MOMS investigators have provided some data that suggests that prenatal surgery may be inappropriate for those fetuses with ventricle size >15mm at the time of screening. These undertakings should be done with methodological rigor.
CONCLUSIONS
Class I evidence from 1 study and Class III evidence from 2 studies suggest that in comparison to postnatal repair, prenatal surgery for MM reduces the risk of developing shunt-dependent hydrocephalus. Therefore, prenatal repair of MM is recommended for those infants who meet specific criteria for prenatal surgery as fetuses to reduce the risk of developing shunt-dependent hydrocephalus (Level I). Differences between prenatal and postnatal repair with respect to the requirement for permanent CSF diversion should be considered alongside other relevant maternal and fetal factors when deciding upon a preferred method of MM closure.
Conflict of Interest
The Guidelines Task Force members were required to report all possible conflicts of interest (COIs) prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Review Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of Task Force Members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methods manuscript.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document.
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
ACKNOWLEDGMENTS
The guidelines task force would like to acknowledge the Congress of Neurological Surgeons Guidelines Committee for their contributions throughout the development of the guideline, the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Guidelines Review Committee, as well as the American Academy of Pediatrics, Child Neurology Society and Spina Bifida Association for their review, comments, and suggestions throughout peer review, as well as the contributions of Trish Rehring, MPH, CHES, Senior Manager of Clinical Practice Guidelines for the CNS, and Gretchen Kuntz, MSW, MLIS, for assistance with the literature searches. Throughout the review process, the reviewers and authors were blinded from one another. At this time the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Kimon Bekelis, MD; Robin Bowman, MD; Timothy J. Brei, MD; Andrew P. Carlson, MD; John Chi, MD; Mark Dias, MD; Jeffrey Olson, MD; John O’Toole, MD; Michael Partington, MD; Curtis Rozzelle, MD; Krystal Tomei, MD; Jan B. Wollack, MD, PhD.
Appendix I. Literature Searches
| PubMed Strategy |
Results |
Embase Strategy |
Results |
Total Results after De-duplication |
| ((((((Meningomyelocele[mh]) OR Meningomyelocele[tw])) AND ((spina bifida[mh]) OR spina bifida[tw]))) AND ((Cerebrospinal Fluid Shunts[mh]) OR Cerebrospinal Fluid Shunts[tw])) AND (((((pediatric[tiab]) OR (infant[mh] OR infant[tw])) OR in utero[tiab]) OR fetal[tw]) OR prenatal[tw]) |
51 |
(('meningomyelocele'/exp OR meningomyelocele) AND ('cerebrospinal fluid shunting'/exp OR 'cerebrospinal fluid shunts') AND ('infant'/exp OR pediatric:ti,ab OR infant OR 'in utero':ti,ab OR fetal OR prenatal) AND ('spinal dysraphism'/exp OR 'spina bifida')) AND [embase]/lim NOT [medline]/lim |
20 |
71 |
Appendix II. PRIMSA Flow Chart
Appendix III: Rating Evidence Quality
Classification of Evidence on Therapeutic Effectiveness
| Class I Evidence Level I Recommendation |
Evidence from one or more well-designed, randomized controlled clinical trial, including overviews of such trials. |
| Class II Evidence Level II Recommendation |
Evidence from one or more well-designed comparative clinical studies, such as non-randomized cohort studies, case-control studies, and other comparable studies, including less well-designed randomized controlled trials. |
| Class III Evidence Level III Recommendation |
Evidence from case series, comparative studies with historical controls, case reports, and expert opinion, as well as significantly flawed randomized controlled trials. |
Appendix IV. Evidence Tables
| Article (Alpha by Author) |
Class of Evidence |
Task Force Conclusions relative to question and rationale for evidence grading |
|
Brunner et al, 19999
|
Class III |
This study provides evidence that intrauterine repair decreases the incidence of shunt-dependent hydrocephalus. This study was a single center non-randomized retrospective review with a minimum follow-up of 6 months. Mothers were allowed to select treatment and the surgical interventions were not standardized. There was significant imbalance in baseline characteristics between the study groups. Specific criteria for shunt placement (the outcome) were not mandated. |
|
Tulipan et al, 201513
|
Class I |
This study provides evidence that compared with postnatal surgery, prenatal surgery for MM performed before 26 weeks of gestation decreased the requirement for CSF shunt placement at 12 months. This study presents the updated 1-year outcomes for the 183 patients who completed MOMS, which was a well-designed, conducted and reported randomized control trial. |
|
Zamlynski et al, 201414
|
Class III |
This study provides evidence that prenatal surgery may decrease the need for CSF shunt placement when compared to postnatal repair of MM. The study was a prospective nonrandomized comparison of outcomes where mothers of children diagnosed with MM who were candidates for either prenatal or postnatal repair were allowed to choose which operation they preferred. Many patients in the postnatal surgery group underwent repairs that were not compliant with study criteria. A significant number of patients were lost to follow-up, leading to concerns regarding ascertainment bias. |
Appendix V. Major Inclusion/Exclusion Criteria of the MOMS12 Trial
| Inclusion |
- Mothers >18 years of age carrying a singleton pregnancy
- Fetuses with normal karyotype
- Fetuses with a gestational age of 19-25.9 weeks at randomization
- Fetuses with MM defect with an upper level between T1 and S1 with evidence of hindbrain herniation
|
| Exclusion |
- Fetuses with a severe kyphotic deformity related to MM
- Fetuses with a fetal anomaly unrelated to MM
- Risk of preterm birth, placental abruption or any other contraindication to surgery.
- Mother with Body Mass Index of >35
|
REFERENCES
- Boulet SL, Yang Q, Mai C, et al. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res. A Clin. Mol. Teratol. Jul 2008;82(7):527-532.
- Hunt GM. 'The median survival time in open spina bifida'. Dev. Med. Child Neurol. Aug 1997;39(8):568.
- Manning SM, Jennings R, Madsen JR. Pathophysiology, prevention, and potential treatment of neural tube defects. Ment Retard Dev Disabil Res Rev. 2000;6(1):6-14.
- Korenromp MJ, van Gool JD, Bruinese HW, Kriek R. Early fetal leg movements in myelomeningocele. Lancet. Apr 19 1986;1(8486):917-918.
- Sival DA, Begeer JH, Staal-Schreinemachers AL, Vos-Niel JM, Beekhuis JR, Prechtl HF. Perinatal motor behaviour and neurological outcome in spina bifida aperta. Early Hum. Dev. Nov 24 1997;50(1):27-37.
- Sutton LN, Adzick NS, Bilaniuk LT, Johnson MP, Crombleholme TM, Flake AW. Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. JAMA. Nov 17 1999;282(19):1826-1831.
- Adzick NS, Sutton LN, Crombleholme TM, Flake AW. Successful fetal surgery for spina bifida. Lancet. Nov 21 1998;352(9141):1675-1676.
- Tulipan N, Bruner JP. Myelomeningocele repair in utero: a report of three cases. Pediatr. Neurosurg. Apr 1998;28(4):177-180.
- Bruner JP, Tulipan N, Paschall RL, et al. Fetal surgery for myelomeningocele and the incidence of shunt-dependent hydrocephalus. JAMA. Nov 17 1999;282(19):1819-1825.
- Tulipan N, Bruner JP, Hernanz-Schulman M, et al. Effect of intrauterine myelomeningocele repair on central nervous system structure and function. Pediatr. Neurosurg. Oct 1999;31(4):183-188.
- Tulipan N, Sutton LN, Bruner JP, Cohen BM, Johnson M, Adzick NS. The effect of intrauterine myelomeningocele repair on the incidence of shunt-dependent hydrocephalus. Pediatr. Neurosurg. Jan 2003;38(1):27-33.
- Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N. Engl. J. Med. Mar 17 2011;364(11):993-1004.
- Tulipan N, Wellons JC, 3rd, Thom EA, et al. Prenatal surgery for myelomeningocele and the need for cerebrospinal fluid shunt placement. J. Neurosurg. Pediatr. Dec 2015;16(6):613-620.
- Zamlynski J, Olejek A, Koszutski T, et al. Comparison of prenatal and postnatal treatments of spina bifida in Poland--a non-randomized, single-center study. J. Matern. Fetal Neonatal Med. Sep 2014;27(14):1409-1417.
- Ransohoff DF, Pignone M, Sox HC. How to decide whether a clinical practice guideline is trustworthy. JAMA. Jan 9 2013;309(2):139-140.
- Rintoul NE, Sutton LN, Hubbard AM, et al. A new look at myelomeningoceles: functional level, vertebral level, shunting, and the implications for fetal intervention. Pediatrics. Mar 2002;109(3):409-413.
- Phillips BC, Gelsomino M, Pownall AL, et al. Predictors of the need for cerebrospinal fluid diversion in patients with myelomeningocele. J. Neurosurg. Pediatr. Aug 2014;14(2):167-172.
- Chakraborty A, Crimmins D, Hayward R, Thompson D. Toward reducing shunt placement rates in patients with myelomeningocele. J. Neurosurg. Pediatr. May 2008;1(5):361-365.
- Steinbok P, Irvine B, Cochrane DD, Irwin BJ. Long-term outcome and complications of children born with meningomyelocele. Childs Nerv. Syst. Mar 1992;8(2):92-96.
- Mirzai H, Ersahin Y, Mutluer S, Kayahan A. Outcome of patients with meningomyelocele: the Ege University experience. Childs Nerv. Syst. Mar 1998;14(3):120-123.
- Bell WO, Sumner TE, Volberg FM. The significance of ventriculomegaly in the newborn with myelodysplasia. Childs Nerv. Syst. 1987;3(4):239-241.
- Danzer E, Finkel R, Gerdes M, et al. The relationship of seizure activity and chronic epilepsy in early infancy and short-term neurodevelopmental outcome following fetal myelomeningocele closure. Neuropediatrics. Jun 2010;41(3):140-143.
- Bruner JP, Tulipan N, Reed G, et al. Intrauterine repair of spina bifida: preoperative predictors of shunt-dependent hydrocephalus. Am. J. Obstet. Gynecol. May 2004;190(5):1305-1312.
- Johnson MP, Sutton LN, Rintoul N, et al. Fetal myelomeningocele repair: short-term clinical outcomes. Am. J. Obstet. Gynecol. Aug 2003;189(2):482-487.
- Johnson MP, Gerdes M, Rintoul N, et al. Maternal-fetal surgery for myelomeningocele: neurodevelopmental outcomes at 2 years of age. Am. J. Obstet. Gynecol. Apr 2006;194(4):1145-1150; discussion 1150-1142.