Guidelines For Pediatric Myelomeningocele
5. The Management of Patients with Myelomeningocele: Whether Persistent Ventriculomegaly Adversely Impacts Neurocognitive Development
Download pdf Neurosurgery, 2019
Sponsored by: Congress of Neurological Surgeons (CNS) and the Section on Pediatric Neurosurgery
Endorsed by: The Congress of Neurological Surgeons (CNS), American Association of Neurological Surgeons (AANS), and Spina Bifida Association (SBA)
Jeffrey P. Blount, MD1, Susan R. Durham, MD MS2, Paul Klimo Jr., MD3, Nadege Assassi4, David F. Bauer, MD5, Alexandra D. Beier, DO6, Ann Marie Flannery, MD7, Catherine McClung-Smith, MD8, Dimitrios C. Nikas, MD9, Patricia Rehring, MPH10, Mandeep S. Tamber, MD, PhD11, Rachana Tyagi, MD12, Catherine A. Mazzola, MD13
- Division of Pediatric Neurosurgery, Department of Neurosurgery, University of Alabama at Birmingham; Children’s of Alabama, Birmingham, Alabama
- University of Vermont, Division of Neurosurgery, Burlington, Vermont
- Semmes-Murphey; Department of Neurosurgery, University of Tennessee Health Science Center; Le Bonheur Children’s Hospital, Memphis, Tennessee
- Department of Surgery, Division of Neurosurgery, Robert Wood Johnson Medical School, New Brunswick, New Jersey
- Department of Surgery, Division of Neurosurgery, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire
- Division of Pediatric Neurosurgery, University of Florida Health Jacksonville, Jacksonville, Florida
- Kids Specialty Center, Women’s & Children’s Hospital, Lafayette, Louisiana
- Department of Neurological Surgery, Palmetto Health USC Medical Group, Columbia, South Carolina
- Division of Pediatric Neurosurgery, Advocate Children's Hospital, Oak Lawn, Illinois
- Congress of Neurological Surgeons, Schaumburg, Illinois
- Division of Pediatric Neurosurgery, British Columbia Children’s Hospital, University of British Columbia, Vancouver, British Columbia, Canada
- Department of Neurosurgery, Mercer University Medical School, Macon, Georgia
- Goryeb Children’s Hospital, Morristown, New Jersey; Rutgers Department of Neurological Surgery, Newark, New Jersey
Correspondence:
Jeffrey P. Blount, MD
University of Alabama Birmingham, Children’s of Alabama
Birmingham, AL
Email: jeffrey.blount@childrensal.org
Abbreviations:
CSF- Cerebrospinal fluid
COI- conflict of interest
ETV- endoscopic third ventriculostomy
CPC- choroid plexus coagulation
LV- lateral ventricle
MM- myelomeningocele
SB- spina bifida
WISC-R- Wechsler Intelligence Scale for Children-Revised
VSI- ventricular surface index
ABSTRACT
Background: Myelomeningocele (MM) is the most common congenital anomaly to affect the nervous system and affects 1500-2000 newborn infants per year in the United States. It is accompanied by symptomatic hydrocephalus in approximately 70-80% of patients. Different treatment strategies for hydrocephalus characteristically result in different effects on the size of the ventricles.
Objective: The objective of this systematic review was to determine whether persistent ventricular enlargement adversely impacts neurocognitive development in patients with MM.
Methods: The PubMed National Library of Medicine Medline database and Embase were queried using MeSH headings and keywords relevant to neurocognitive or intellectual development and ventricular size or morphology. Abstracts were reviewed by the authors to identify which studies met strict inclusion criteria. An evidence table was constructed that summarized the included studies and reflected the quality of evidence (Classes I–III) that each represented. A recommendation was made that is based on the quality of the evidence.
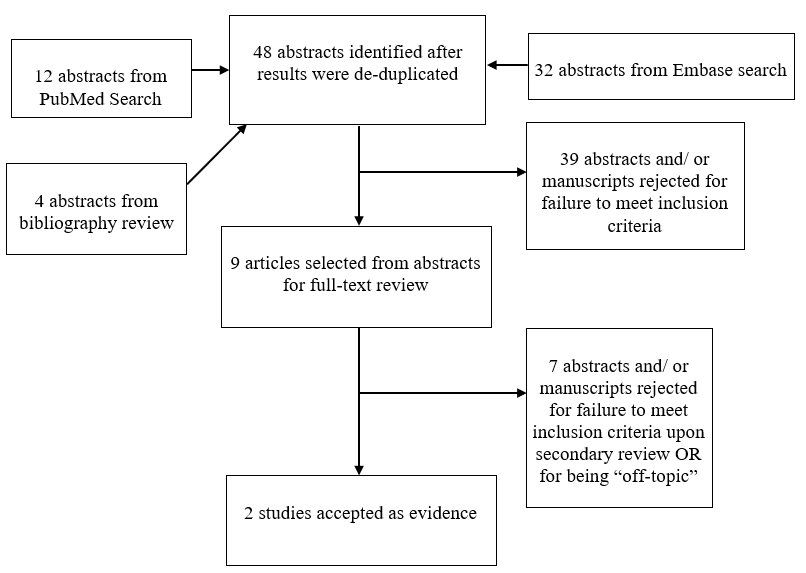
Results: An initial abstract review utilizing strict inclusion/exclusion criteria yielded 48 studies, 9 of which underwent full-text review. There is limited and conflicting Class III evidence from 2 studies.
Conclusions: Currently, there is insufficient data to conclude that ventricular size and morphology impact neurocognitive development.
Keywords: Cognition, development, fetal, hydrocephalus, in utero, myelomeningocele, ventriculomegaly
RECOMMENDATIONS
PICO Question: In myelomeningocele patients with hydrocephalus, does persistent enlargement of the ventricles adversely impact neurocognitive development?
Target Population: Myelomeningocele patients with hydrocephalus.
Recommendation: Currently, there is insufficient data to conclude that ventricular size and morphology impact neurocognitive development.
INTRODUCTION
Hydrocephalus requiring surgical intervention occurs in 70-80% of children with myelomeningocele (MM).1 Prior to the 1960s, there were no consistently effective treatments for MM and the vast majority of patients with MM did not survive due to untreated hydrocephalus.2 The development of non-reactive silastic (rubberized silicone) shunt tubing and the development of functional shunt valves enabled the creation of ventricular shunts, which have since been the mainstay of treatment. Shunts drain the cerebrospinal fluid (CSF) from the ventricles, to another body cavity (typically the peritoneum or the heart/atrium) where it can be re-absorbed. Shunts have been highly effective in the treatment of hydrocephalus, but are also plagued by high morbidity due to shunt blockage, shunt infection, shunt fracture, disconnection or shunt over-drainage. Despite innovation and extensive trials of improving shunt design, these complications of shunting occur at significant levels at virtually all centers that provide care. Furthermore, shunts are not available in all countries, due to cost and limited availability.
Due to these challenges, a variety of alternate treatments for symptomatic hydrocephalus in patients with MM have been proposed. Some centers advocate a less aggressive approach to the treatment of patients with ventriculomegaly associated with MM.3, 4 Infants with asymptomatic ventriculomegaly are closely monitored for progressive macrocephaly, signs of neurological dysfunction and/ or progressive ventricular enlargement. In the absence of these concerns, children with asymptomatic ventriculomegaly may not be offered surgical treatment and are often managed conservatively.
Another important alternative treatment is an endoscopic third ventriculostomy (ETV) with or without choroid plexus coagulation (CPC).5 This innovative approach was developed by Warf and has gained attention and popularity due to initial indications of good efficacy (in cohorts of children in Uganda and smaller cohorts in North America).6 Follow up studies in North American prospective cohorts have shown that ETV with CPC has a mildly higher failure rate of hydrocephalus control than shunts.5, 7 However, ETV with CPC has retained its popularity due to its freedom from shunt dependency (with associated high shunt failure morbidity) and reduced cost. One characteristic of ETV with CPC is that the ventricles often remain enlarged even in procedures that are clinically effective at reducing raised intracranial pressure.
These recent management strategies of infants with MM often result in persistent ventriculomegaly. The long-term effects of chronic ventriculomegaly on neurocognitive development in these infants have not been determined.
OBJECTIVES
This systematic review seeks to determine whether there is evidence that persistent ventriculomegaly or other morphologic characteristics of the peri-ventricular spaces correlate with impairment of normal neurocognitive development. Neurocognitive development in this context refers to the normal development of age-appropriate learning, memory and attendant social and behavioral skills and contrasts this with impairments in these functions that arise from acute or chronic brain injury or illness. This guideline can support clinical decision making or counseling regarding available treatment options for infants with MM. Intended users of these guidelines include professionals who provide medical or surgical treatment or consultation to families with children with MM.
METHODS
Writing Group and Question Establishment
The Guidelines Task Force initiated a systematic review of the literature and evidence-based guideline relevant to the diagnosis and treatment of patients with MM. Through objective evaluation of the evidence and transparency in the process of making recommendations, this evidence-based clinical practice guideline was developed for the diagnosis and treatment of patients with MM. These guidelines are developed for educational purposes to assist practitioners in their clinical decision-making processes. Additional information about the methods utilized in this systematic review is provided in the introduction and methodology chapter.
Literature Search
The task force members collaborated with a medical librarian to search the PubMed National Library of Medicine Medline database and Embase Database for the period from 1966 to September 2016 using the search strategies provided in Appendix I. The literature search yielded 44 original papers. An additional bibliography search of these candidate papers revealed an additional 4 candidate papers resulting in 48 total abstracts. Based on inclusion/exclusion criteria detailed below, the task force selected 9 articles for full review. Seven8-14 of these were rejected for not meeting inclusion criteria or for being off-topic.
A series of authors for the development of guidelines related to MM were identified and screened for conflict of interest. This group, in turn, agreed on a set of pertinent questions to address the topic at hand, and conducted a systematic review of the literature relevant to MM. Additional details of the systematic review are provided below and within the introduction and methodology chapter of the guideline.
The recommendations deliberately eschewed the use of expert opinion, and instead relied strictly on the available literature.
Study Selection and Eligibility Criteria
Utilizing the MESH criteria outline below, authors identified 44 candidate papers (after de-duplication) that addressed neurocognitive outcomes in hydrocephalus related to MM. A bibliography search of these candidate papers revealed an additional 4 candidate papers, yielding 48 abstracts in total. The abstract for each article was evaluated by 3 separate independent reviewers and a determination made whether the topic and study design pertained to the research question. The 3 sets of data were compared for agreement by the other members of the task force. There was near uniform opinion with regard to inclusion of articles and minor differences were resolved by consensus. A total of 39 papers were excluded. To be included in this guideline, an article must be a report of a study that:
- At least 80% of patients had to be patients with MM and <18 years of age.
- Studies that enrolled >20% of patients with other forms of spina bifida (SB) were excluded.
- Studies that combined the results of patients with other forms of SB were excluded if the study enrolled less than 80% of target patient population.
- Studies that enrolled mixed patient populations were included only if they reported separate results for the target population. The results of the target population were the only results considered as evidence to support our recommendations.
- The study was a full article report of a clinical study.
- The study was not a meeting abstract, editorial, letter, or a commentary.
- Prospective case series had to report baseline values, if applicable.
- Case series studies with non-consecutive enrollment of patients were excluded.
- Studies had to have appeared in a peer-reviewed publication or a registry report.
- Studies had to enroll at least 10 patients for each distinct outcome measured. If it was a comparative study, a minimum enrollment of 5 patients per treatment arm for each outcome was necessary.
- The study involved humans.
- The study was published between January 1966 and September 2016.
- The study presented results quantitatively.
- The study did not involve “in vitro”, “biomechanical” or results performed on cadavers.
- The study was published in English.
- Papers reporting results of systematic reviews, meta-analyses, or guidelines developed by others were excluded.
- Authors specifically excluded follow-up studies in which a cohort of patients from an initial study were followed in time and separately reported upon in a subsequent publication. This prevented the same patients from being included multiple times in this review.
The authors did not include systematic reviews, guidelines, or meta-analyses conducted by others. These documents were developed using different inclusion criteria than those specified in this guideline. Therefore, they may include studies that do not meet the inclusion criteria specified above. The bibliographies of these papers were searched for additional studies.
There were 2 studies6, 15 that met inclusion criteria (see Appendix IV).
Data Collection Process
The abstracts that met the selection criteria mentioned above were retrieved in full-text form. Each article’s adherence to the selection criteria was confirmed. To determine how the data could be classified, the information in the full-text articles was then evaluated to determine whether they were providing results of therapy or were more centered on diagnostic or prognostic information. Agreement on these assessments and on the salient points regarding the type of study design and objectives, and the conclusions and data classification was then reached by exchanging drafts and comments by e-mail and discussing questions during monthly phone conference among participants. The information was then used for construction of the evidence tables.
Assessment for Risk of Bias
The literature included in full text review was assessed for risk of bias. Bias may be a problem given the small number of references and the study design of each report. Each of the papers that met the inclusion criteria was a retrospective review. In the study performed by Warf et al,6 the data was prospectively collected but analysis was retrospective, which resulted in class III evidence. Reporting bias is a potential risk. Other potential contributions to bias include bias due to selective case choice or loss of information over time is also noted. Other potential sources in this survey include publication bias and hidden agenda bias. Warf is the developer of contemporary techniques in ETV/CPC. As such there is potential for bias to impact this paper and therefore, due to the nominal number of available studies, the guideline.
Rating Quality of Evidence
The quality of evidence was rated using an evidence hierarchy for therapeutic, prognostic, diagnostic, and decision modeling. These hierarchies are shown in Appendix III: Rating Evidence Quality. Additional information regarding the hierarchy classification of evidence can be located here on the CNS Guidelines methodology page.
Revision Plans
In accordance with the Institute of Medicine’s standards for developing clinical practice guidelines, the task force will monitor related publications following the release of this document and will revise the entire document and/or specific sections “if new evidence shows that a recommended intervention causes previously unknown substantial harm; that a new intervention is significantly superior to a previously recommended intervention from an efficacy or harms perspective; or that a recommendation can be applied to new populations.”16 In addition, the task force will confirm within 5 years from the date of publication that the content reflects current clinical practice.
RESULTS
Study Selection and Characteristics
The literature review identified 9 studies that were potentially useful in providing primary data to address the issue of the impact of ventriculomegaly on neurocognitive development. Seven of these studies were excluded because they either failed to align with the objective of this guideline, were review papers or they surveyed neurocognitive development in patients with hydrocephalic MM, but failed to correlate with ventricular measurements.
Results of Individual Studies, Discussion of Study Limitations and Risk of Bias
The relationship between ventricular size in treated symptomatic hydrocephalus and neurocognitive development is unknown. While there are several studies that address outcomes of hydrocephalus in SB there are very few studies that correlate ventricular size with neurocognitive development. All studies that directly address these variables are class III.6, 15 Warf et al6 utilized the Bayley’s Scale of Infant Development Version 3 (BSID III) as an assessment of cognitive development in a 93 patient cohort of east African children. Each child had MM-related hydrocephalus and underwent 1 of 3 forms of treatment. Group 1 (n=53) underwent ETV with CPC while Group 2 (n=19) had a ventricular shunt placed. The third group required no treatment. BSID III examinations were adjusted to be culturally accurate (average age of testing=15.6 months). Linear regression analysis was performed for each BSID component score utilizing the BSID as the dependent variable and the frontal occipital ratio as the independent variable. Patients in group 3 who did not develop hydrocephalus fared the best but there was no significant difference observed between groups of children with hydrocephalus regardless of treatment paradigm (ETV with CPC vs shunt). No longitudinal observation was pursued.
Fletcher et al studied neurocognitive outcome in hydrocephalus17 and have multiple papers that address a growing cohort of patients followed over an extended period.18, 19 While this group addresses the issues concerned in this guideline none of their studies can be included because of failure to meet one of more of the inclusion criteria specified. As a result these papers were not included in evidence but their findings and contributions are briefly considered in the discussion section.
Ito et al15 observed that there were very few studies that correlated intellectual capability and ventricular size and morphology. In a small cohort of 12 shunted patients with SB they observed that shunted patients with MM demonstrated preferential distention of the posterior horns of the ventricles and the overlying parieto-occipital regions. They hypothesized that visuospatial capabilities would be selectively involved. Utilizing the Wechsler Intelligence Scale for Children-Revised (WISC-R) standardized measure of intellectual capability, each patient’s verbal IQ (V-IQ) and performance IQ (P-IQ) was determined. The morphology of the ventricles was assessed utilizing a computerized image analyzer of the axial sequences of T2 weighted MRI scans. From these measurements, the ventricular surface index (VSI) was defined as the ratio of the ventricular surface to the whole brain surface at the same level. The anterior ventricular morphology was described as VSI (A) which reflected the ratio at the anterior extent of the frontal horns while the VSI (P) reflected parieto-occipital morphology. There was no difference observed in the anterior measurements between hydrocephalic patients and normal controls. This observation corroborated the hypothesis that in their cohort of shunted SB patients, there was selective dilation of the posterior horns. They then examined the correlation between the morphologic index and perceptual disability with particular regard for visual-spatial processing. They found a highly significant correlation between the dilation of posterior horns of the ventricles and the extent of visuospatial processing disturbances. Limitations of this study were its small sample size, non-validated metrics and retrospective methodology.
DISCUSSION
Within the limits of this systematic review, the authors found conflicting Class III evidence with regard to the relationship between ventricular size and volume and neurocognitive development and the relationship is currently unknown. More research is needed to better determine the effect of ventricular size and volume on neurocognitive development in children with hydrocephalus from MM.
The impact of ventricular size and morphology on the neurocognitive development of children with MM has received limited attention in the literature until recently. Researchers who previously studied the neurocognitive outcomes of patients with hydrocephalus utilized single institution retrospective observational cohort methodology. There is very limited sub-stratification of patients by diagnosis with correlation for ventricular characteristics. As a result, there is very limited information available about ventricular size and morphology and its correlation with learning and development. However, the relationship between ventricular distention and impact on cognition matters because there is a growing clinical preference toward approaches to treatment of hydrocephalus that may result in persistent ventriculomegaly. The definition of a satisfactory clinical outcome for treatment of hydrocephalus is evolving. Traditionally the placement of a shunt has resulted in restoration of normal, small or slit-like ventricles. As a result, normal or small ventricles were the most frequent result of intervention and were considered central to successful treatment of hydrocephalus. Over the last 10-15 years 2 trends have emerged that challenge this central dictum of hydrocephalus treatment and place the relationship between ventricular size and cognition at the forefront of hydrocephalus controversy. The first trend is to increase the treatment threshold for placement of a shunt by tolerating larger ventricles and performing more local wound care for CSF leaks following MMC closure. This approach has been advocated by Thompson in London and Bowman in Chicago and has resulted in significant reductions in shunt placement rates.3, 4 The second is the widespread embrace of ETV/CPC as a preferred technique for treatment of hydrocephalus.5 Both of these approaches spare the child placement of a shunt and thereby prevent downstream chronic shunt problems and morbidity. However, they are also typically associated with persistent ventriculomegaly. At present there exists only limited data to support that these approaches do not threaten normal neurocognitive development. If enlarged ventricles impair cognitive growth it becomes increasingly difficult to justify any clinical approach that would leave the ventricles stretched and distended when an alternative treatment is available that adequately decompresses them and results in small or normal ventricles.
Traditionally ventricular characteristics have served as a proxy metric that describes the adequacy of the treatment of hydrocephalus. Fundamentally researchers have not identified the critical variable that most favorably impacts neurocognitive development. Ventricular size has been utilized primarily because it is readily measured (either radiographically or utilizing head circumference as a proxy in young children) and conceptually attractive.
It is intuitively consistent that pronounced distention of white matter fibers and associated thinning of the overlying cortex could be detrimental to a process so refined and complex as human learning. Multiple morphometric studies that examine the histologic morphology of neurons in the brain of hydrocephalic animals demonstrated improvements in neuronal structural morphology following placement of a shunt.20, 21 Whether these improvements arose from dissipation of pressure or distention remains unknown. Several contemporary models of hydrocephalus focus more on pulsatility, brain elasticity and compliance and the physical potential for the nervous system to serve as a capacitor for imparted physical forces. Such models have gained increasing acceptance amongst some experienced centers. Such models consider simple drainage via ventricular shunts a negative force that progressively adversely affects critical visco-elastic and compliance qualities of the developing brain. In such models, the critical variables are those of pulsatility, elasticity and compliance and ventricular morphology and size is a minimal consideration.
The most comprehensive study that specifically addresses the issue of ventriculomegaly in hydrocephalic patients with MM was published by Warf et al6 in 2009. Retrospective analysis of prospectively collected data on a cohort of 93 infants with MMC induced hydrocephalus from Uganda revealed similar neuro-cognitive outcomes in cohorts of patients treated with ETV/CPC and VP shunt. Treatment group assignment was not randomized and reflected clinical need. The metric for cognitive assessment was the BSID III. While follow up and compliance were exceptional, the limitations of the study include retrospective observational cohort design, limited sample numbers, limited follow up time, young age of testing and lack of longitudinal follow up. The BSID III metric to assess neurocognitive development may or may not be appropriate for the cohort in which it was utilized. The geographic and cultural disparity between this cohort and other cohorts challenge potential application of the data broadly. Despite limitations to the power of this study it continues to provide best for available data to address neuro-cognitive development in cohorts of patients with MM.
In 1992, Fletcher and colleagues reported on cognition and brain white matter changes in a cohort of 23 patients with hydrocephalus.17 There were 32 hydrocephalic patients studied of whom 17 had open MM as an etiology and 6 had meningocele. Even if the meningocele patients are included as dysraphic then only 72% (23/32) of the cohort has dysraphism and the inclusion criteria for this guideline of 80% is not met. However, this cohort is of substantive size and only marginally misses the inclusion criteria. The authors found no correlation with verbal learning and ventricular size but found significant correlation with both verbal and non-verbal learning with volume loss in the adjacent corpus callosum. Non-verbal learning also correlated with ventricular size in the right but not left lateral ventricle (1). However, these findings as well as the study overall finding of lower IQ with larger ventricles cannot be included in evidence due to failure to meet the inclusion criteria.
In a related paper Fletcher et al9 retrospectively correlated ventricular size and morphology in a cohort of 99 patients with hydrocephalus by utilizing MRI measurements for ventricular size and the WISC-R validated instrument to measure verbal and performance IQ. This study was excluded because only 40/99 (40%) patients had SB as the etiology of the hydrocephalus. As such it failed to meet inclusion criteria for analysis which required 80% of patients within the cohort have hydrocephalus from MM. The authors found that the ratio of the lateral ventricle (LV) posterior horn: (LV) anterior horn was negatively correlated with visuospatial ability but this cannot be considered as evidence for this guideline. A subsequent publication by this group evaluated 88 patients with hydrocephalus but none were due to MM and as a result this paper was excluded as well.
Based on the paucity of data available, there is insufficient evidence to make a recommendation on whether ventriculomegaly adversely affects neurocognitive development in children with MM.
CONCLUSIONS
The conclusion of this systematic review is with such incomplete data available, the results are mixed from a very limited number of studies and there is insufficient evidence to make a recommendation.
Future Research
Treatment of hydrocephalus is central to the care of children with MM. The most widely utilized current treatment is placement of a ventricular shunt. In the majority of cases this is effective but may place the child at risk for significant resultant morbidity from shunt obstruction and shunt infection. Treatment alternatives such as ETV with CPC and raising the threshold criteria for shunt placement are being advocated and evaluated. Each of these treatments may be associated with persistent ventricular enlargement after treatment. Future studies need to be directed at prospectively studying neurocognitive development with validated instrument assays in patients with MM utilizing validated neuro-cognitive instruments and longitudinal follow up to correlate developmental outcome with ventricular size and brain volume.
Conflict of Interest
The Guidelines Task Force members were required to report all possible conflicts of interest (COIs) prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Review Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of Task Force Members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methodology manuscript.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document.
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
ACNKOWLEDGMENTS
The guidelines task force would like to acknowledge the Congress of Neurological Surgeons Guidelines Committee for their contributions throughout the development of the guideline, the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Guidelines Review Committee, as well as the American Academy of Pediatrics, Child Neurology Society and Spina Bifida Association for their review, comments, and suggestions throughout peer review, as well as the contributions of Trish Rehring, MPH, CHES, Senior Manager of Clinical Practice Guidelines for the CNS, and Gretchen Kuntz, MSW, MLIS, for assistance with the literature searches. Throughout the review process, the reviewers and authors were blinded from one another. At this time the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Kimon Bekelis, MD; Robin Bowman, MD; Timothy J. Brei, MD; Andrew P. Carlson, MD; John Chi, MD; Mark Dias, MD; Jeffrey Olson, MD; John O’Toole, MD; Michael Partington, MD; Curtis Rozzelle, MD; Krystal Tomei, MD; Jan B. Wollack, MD, PhD.
Appendix I. Literature Search Terms
| PubMed Strategy |
Results |
Embase Strategy |
Results |
Total Results after De-duplication |
| (((((((Hydrocephalus[mh]) OR Hydrocephalus[tw]) OR ventriculomegaly[tw])) AND (spina bifida[mh] OR spina bifida[tw]))) AND ((((((((Cognition[mh]) OR Cognition[tw]) OR Memory[mh]) OR Memory[tw]) OR Executive Function[mh]) OR Executive Function[tw]) OR Educational Status[mh]) OR Educational Status[tw])) AND (infant[mh] OR infant[tw] OR pediatric[tiab]) |
12 |
(('spinal dysraphism'/exp OR 'spina bifida') AND ('hydrocephalus'/exp OR hydrocephalus OR ventriculomegaly) AND ('cognition'/exp OR cognition OR 'memory'/exp OR memory OR 'executive function'/exp OR 'executive function' OR 'educational status'/exp OR 'educational status') AND (('infant'/exp OR infant) OR pediatric:ti,ab)) AND [embase]/lim NOT [medline]/lim |
32 |
44 |
Appendix II. PRISMA Article Flow Chart
Appendix III: Rating Evidence Quality
Classification of Evidence on Therapeutic Effectiveness
| Class I Evidence Level I Recommendation |
Evidence from one or more well-designed, randomized controlled clinical trial, including overviews of such trials. |
| Class II Evidence Level II Recommendation |
Evidence from one or more well-designed comparative clinical studies, such as non-randomized cohort studies, case-control studies, and other comparable studies, including less well-designed randomized controlled trials. |
| Class III Evidence Level III Recommendation |
Evidence from case series, comparative studies with historical controls, case reports, and expert opinion, as well as significantly flawed randomized controlled trials. |
Appendix IV. Evidence Tables
| Article (Alpha by Author) |
Class of Evidence |
Task Force Conclusions relative to question and rationale for evidence grading |
|
Ito et al, 199715
|
Class III |
Ito and colleagues retrospectively correlated ventricular size and morphology in a cohort of 12 patients with SB related hydrocephalus by utilizing MRI measurements for ventricular size and the WISC-R validated instrument to measure verbal and PIQ. They found that the ratio of the LV posterior horn: LV anterior horn was negatively correlated with visuospatial ability. Tools used to study follow up include: WISC-R and selective Frostig DTVP. |
|
Warf et al, 20096
|
Class III |
This study included a 93 patient cohort of East African children. The data was prospectively collected but the analysis was retrospective. This study showed no evidence for correlation between ventricular size and cognitive outcome. Tools used to study follow up include: Modified BSID III. |
BSID, Bayley’s Scale of Infant Development; SB, spina bifida; MRI, magnetic resonance imaging; LV, lateral ventricle; DTVP, Developmental Test of Visual Perception; WISC-R, Wechsler Intelligence Scale for Children-Revised; PIQ, Performance Intelligence Quotient
REFERENCES
- Warf BC, Alkire BC, Bhai S, et al. Costs and benefits of neurosurgical intervention for infant hydrocephalus in sub-Saharan Africa. J. Neurosurg. Pediatr. Nov 2011;8(5):509-521.
- Lorber J. Results of treatment of myelomeningocele. An analysis of 524 unselected cases, with special reference to possible selection for treatment. Dev. Med. Child Neurol. Jun 1971;13(3):279-303.
- Chakraborty A, Crimmins D, Hayward R, Thompson D. Toward reducing shunt placement rates in patients with myelomeningocele. J. Neurosurg. Pediatr. May 2008;1(5):361-365.
- Norkett W, McLone DG, Bowman R. Current Management Strategies of Hydrocephalus in the Child With Open Spina Bifida. Top Spinal Cord Inj Rehabil. Fall 2016;22(4):241-246.
- Stone SS, Warf BC. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment for infant hydrocephalus: a prospective North American series. J. Neurosurg. Pediatr. Nov 2014;14(5):439-446.
- Warf B, Ondoma S, Kulkarni A, et al. Neurocognitive outcome and ventricular volume in children with myelomeningocele treated for hydrocephalus in Uganda. J. Neurosurg. Pediatr. Dec 2009;4(6):564-570.
- Kulkarni AV, Riva-Cambrin J, Rozzelle CJ, et al. Endoscopic third ventriculostomy and choroid plexus cauterization in infant hydrocephalus: a prospective study by the Hydrocephalus Clinical Research Network. J. Neurosurg. Pediatr. Mar 2018;21(3):214-223.
- Erickson DV. Mental functioning of infants with spina bifida on the Bayley Scales of Infant Development. Canadian Journal of Rehabilitation. 1990;3(3):159-166.
- Fletcher JM, Bohan TP, Brandt ME, et al. Morphometric evaluation of the hydrocephalic brain: relationships with cognitive development. Childs Nerv. Syst. Apr 1996;12(4):192-199.
- Lomax-Bream LE, Barnes M, Copeland K, Taylor HB, Landry SH. The impact of spina bifida on development across the first 3 years. Dev. Neuropsychol. 2007;31(1):1-20.
- Mataro M, Junque C, Poca MA, Sahuquillo J. Neuropsychological findings in congenital and acquired childhood hydrocephalus. Neuropsychol. Rev. Dec 2001;11(4):169-178.
- Özdemir SA, Özdemir N, Erol SÖ, Ünal VM, Özer EA. Outcomes of neonates with meningomyelocele: Single institute experience. Childs Nerv. Syst. 2015;31(10):1978.
- Rocque BG, Bishop ER, Scogin MA, et al. Assessing health-related quality of life in children with spina bifida. J. Neurosurg. Pediatr. Feb 2015;15(2):144-149.
- Muñoz Marrón E. Neuropsychological profile in spina bifida and hidrocephalus. Mapfre Medicina. 2007;18(SUPPL. 1):102-113.
- Ito J, Saijo H, Araki A, et al. Neuroradiological assessment of visuoperceptual disturbance in children with spina bifida and hydrocephalus. Dev. Med. Child Neurol. Jun 1997;39(6):385-392.
- Ransohoff DF, Pignone M, Sox HC. How to decide whether a clinical practice guideline is trustworthy. JAMA. Jan 9 2013;309(2):139-140.
- Fletcher JM, Bohan TP, Brandt ME, et al. Cerebral white matter and cognition in hydrocephalic children. Arch. Neurol. Aug 1992;49(8):818-824.
- Dennis M, Landry SH, Barnes M, Fletcher JM. A model of neurocognitive function in spina bifida over the life span. J. Int. Neuropsychol. Soc. Mar 2006;12(2):285-296.
- Williams VJ, Juranek J, Stuebing KK, et al. Postshunt lateral ventricular volume, white matter integrity, and intellectual outcomes in spina bifida and hydrocephalus. J. Neurosurg. Pediatr. Apr 2015;15(4):410-419.
- Del Bigio MR, Bruni JE. Cerebral water content in silicone oil-induced hydrocephalic rabbits. Pediatr. Neurosci. 1987;13(2):72-77.
- Del Bigio MR. Neuropathological changes caused by hydrocephalus. Acta Neuropathol. 1993;85(6):573-585.