Reposted with permission from ©AANS, 2014
J Neurosurg Pediatrics (Suppl) 14:60–71, 2014
AANS, 2014
(Original text of the guideline was edited to reflect the update. Please click here for the original publication.)
Pediatric hydrocephalus: systematic literature review and evidence-based guidelines
Part 8: Management of cerebrospinal fluid shunt infection
UPDATE
Mandeep S. Tamber, MD,1 Paul Klimo, Jr., MD, MPH,2,3 Catherine A. Mazzola, MD,4 Ann Marie Flannery, MD5
1Department of Pediatric Neurological Surgery, Children’s Hospital of Pittsburgh, University of Pittsburgh, Pittsburgh, Pennsylvania; 2Department of Neurosurgery, University of Tennessee Health Science Center, Memphis, and 3Le Bonheur Children’s Hospital, Memphis, Tennessee; 4Division of Pediatric Neurological Surgery, Goryeb Children’s Hospital, Morristown, New Jersey; and 5Department of Neurological Surgery, Saint Louis University, St. Louis, Missouri
Object. The objective of this systematic review was to answer the following question: What is the optimal treatment strategy for CSF shunt infection in pediatric patients with hydrocephalus?
Methods. The US National Library of Medicine and the Cochrane Database of Systematic Reviews were queried using MeSH headings and key words relevant to the objective of this systematic review. Abstracts were reviewed, after which studies meeting the inclusion criteria were selected and graded according to their quality of evidence (Classes I–III). Evidentiary tables were constructed that summarized pertinent study results, and based on the quality of the literature, recommendations were made (Levels I–III).
Results. A review and critical appraisal of 27 studies that met the inclusion criteria allowed for a recommendation for supplementation of antibiotic treatment using partial (externalization) or complete shunt hardware removal, with a moderate degree of clinical certainty. However, a recommendation regarding whether complete shunt removal is favored over partial shunt removal (that is, externalization) could not be made owing to severe methodological deficiencies in the existing literature. There is insufficient evidence to recommend the use of intrathecal antibiotic therapy as an adjunct to systemic antibiotic therapy in the management of routine CSF shunt infections. This also holds true for other clinical scenarios such as when an infected CSF shunt cannot be completely removed, when a shunt must be removed and immediately replaced in the face of ongoing CSF infection, or when the setting is ventricular shunt infection caused by specific organisms (for example, gram-negative bacteria).
Conclusions. Supplementation of antibiotic treatment with partial (externalization) or complete shunt hardware removal are options in the management of CSF shunt infection. There is insufficient evidence to recommend either shunt externalization or complete shunt removal as the preferred surgical strategy for the management of CSF shunt infection. Therefore, clinical judgment is required. In addition, there is insufficient evidence to recommend the combination of intrathecal and systemic antibiotics for patients with CSF shunt infection when the infected shunt hardware cannot be fully removed, when the shunt must be removed and immediately replaced, or when the CSF shunt infection is caused by specific organisms. The potential neurotoxicity of intrathecal antibiotic therapy may limit its routine use.
Recommendation: Supplementation of antibiotic treatment with partial (externalization) or with complete shunt hardware removal is an option in the management of CSF shunt infection. Strength of Recommendation: Level II, moderate degree of clinical certainty.
Recommendation: There is insufficient evidence to recommend either shunt externalization or complete shunt removal as a preferred surgical strategy for the management of CSF shunt infection. Therefore, clinical judgment is required. Strength of Recommendation: Level III, unclear degree of clinical certainty.
Recommendation: There is insufficient evidence to recommend the combination of intrathecal and systemic antibiotics for patients with CSF shunt infection in whom the infected shunt hardware cannot be fully removed or must be removed and immediately replaced, or when the CSF shunt infection is caused by specific organisms. The potential neurotoxicity of intrathecal antibiotic therapy may limit its routine use. Strength of Recommendation: Level III, unclear degree of clinical certainty.
(http://thejns.org/doi/abs/10.3171/2014.7.PEDS14328)
Key Words: cerebrospinal fluid shunt, infection, therapy, pediatrics, evidence-based guidelines, practice guidelines, hydrocephalus
Abbreviations used in this paper: EVD = external ventricular drain; VA = ventriculoatrial; VP = ventriculoperitoneal.
Cerebrospinal fluid shunt infection is one of the most common and serious complications of CSF shunt therapy. Infection admissions number approximately 2300 per year in the United States and, in aggregate, account for more than 50,000 hospital days.1 Total hospital charges related to the management of CSF shunt infection were nearly $250 million in 2003 adjusted dollars.1
Within 24 months after insertion, infections complicate approximately 11% of initial CSF shunt placements.2 Despite the high incidence of this complication, the optimal management of CSF shunt infection has yet to be defined. The existing evidence regarding the management of CSF shunt infection is of poor methodological quality. As such, current management is dictated not by evidence, but rather by physician preference and other possibly relevant patient-level factors (for example, patient surgical risk, ventricle size, and complexity of the shunt system). It is not surprising that there is significant variation in CSF shunt infection treatment protocols between centers.3
The objective of this systematic review was to answer the following question: What is the optimal treatment strategy for CSF shunt infection in pediatric patients with hydrocephalus? The successful treatment of CSF shunt infection aims to cure the infection (that is, minimize the probability of reinfection or relapse) while maintaining functional CSF diversion and minimizing morbidity, mortality, and the cost of therapy. The alternative paradigms for the management of ventricular shunt infection are illustrated well if one considers important historical milestones in the treatment of hydrocephalus. The evidentiary tables are structured somewhat accordingly (Fig. 1). The development of the Holter-Pudenz valve in 1957 and the ability to insert the distal end of a ventricular shunt into the right atrium was a major development in the treatment of hydrocephalus. Although ventriculoatrial (VA) shunts facilitated continuous and regulated CSF diversion, the fact that the distal catheter entered the heart posed logistical problems when these shunts inevitably became infected. A major issue with VA shunts was loss of limited venous access if these shunts were removed and not immediately replaced. In light of this limitation, the predominance of literature examining the treatment of CSF shunt infections in the era of VA shunts documented the outcomes of treatment with systemic antibiotics alone (Table 1) and whether the elevated CSF antibiotic concentrations achieved by intrathecal therapy conferred any additional benefit in managing the ventriculitis that often accompanied CSF shunt infection—both while leaving the infected shunt in situ or after removing the shunt and immediately replacing it in infected cerebrospinal fluid (Table 2).
A decade later, Ames developed a technique for placement of the distal catheter in the peritoneal space, and as such, made shunt removal and later replacement a feasible surgical strategy in the management of CSF shunt infection. Over time, the combined medical and surgical treatment of ventricular shunt infection became more accepted, in part because of the gradual phase-out of VA shunts and their associated limitations with respect to repeated surgical access to the heart, but perhaps more significantly because of the realization that an infected ventricular shunt, as an infected foreign body, was difficult if not impossible to sterilize using antibiotics alone. This management philosophy accepts not only that shunt removal (and eventual replacement once CSF sterility is achieved) requires multiple surgeries, but also the risk of introducing secondary infection during a variable period of external drainage. Therefore, although more contemporary literature examining the treatment of CSF shunt infection consists of studies that incorporate some form of shunt removal, variations in whether the infected shunt was partially removed (that is, externalized) (Table 3) or completely removed (see Table 4), and whether supple- mental intrathecal antibiotics were administered contribute to significant between-study heterogeneity.
A lack of rigorous comparative effectiveness studies leads to uncertainty regarding the preferred therapeutic strategy for a particular clinical circumstance. Decision analytical modeling attempts to apply statistical simulation techniques to preexisting data to rank competing therapeutic options in terms of their relative effective- ness. A decision analysis examining the treatment of CSF shunt infection using data from published studies (most included in evidentiary Tables 1–4) came to the conclusion that the best treatment modality for CSF shunt infection was antibiotic administration (systemic, with or without intrathecal administration) and complete removal of the infected shunt, with intercurrent external ventricular drainage or ventricular taps, followed by placement of a new shunt when CSF sterility is achieved. Sensitivity analyses revealed that this treatment option had the highest cure rate, the lowest failure rate, and the lowest mortality rate when compared with treatment consisting of antibiotic therapy with shunt removal and immediate replacement, or antibiotic treatment alone, over a wide range of assumptions.4
Multiple review articles on the topic also conclude that shunt infection should be ideally managed with antibiotics, complete shunt removal, and placement of a temporary external ventricular drain (EVD), followed by reimplantation after CSF sterilization.5-9 Although intrathecal administration of antibiotics appears to make theoretical sense because of enhanced CSF antibiotic concentrations, its practical application is controversial, owing in large part to the potential adverse effects of intrathecal therapy, including neurotoxicity. The indications for intrathecal therapy are not well established and presently range from use in any shunt infection, use in only those infections in which the CSF cannot be sterilized by systemic antibiotics alone (for example, persistent positive cultures), or use in those ventricular shunt infections caused by specific organisms (for example, gram- negative infections). A practice survey of board-certified members of the American Society of Pediatric Neurosurgeons revealed that most surgeons treat ventricular shunt infection with antibiotics, removal of the infected CSF shunt, and placement of an EVD, followed by delayed shunt replacement—a management paradigm that can be supported by the available evidence, as detailed below.
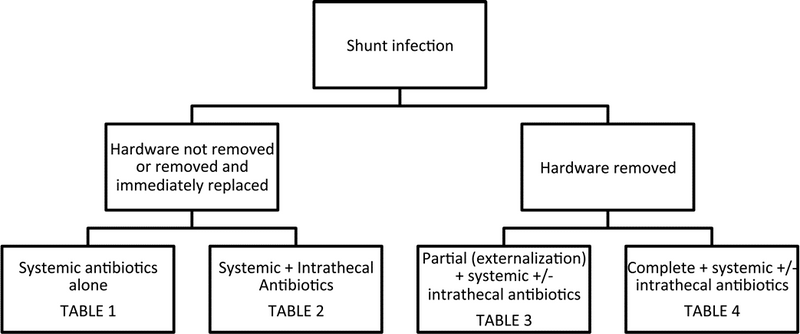
Fig. 1. Organization of evidentiary tables based on alternative paradigms for the management of CSF shunt infection.
Methods
Search Criteria
We searched the US National Library of Medicine (PubMed/MEDLINE) database and the Cochrane Data- base of Systematic Reviews for the period January 1966 through March 2012 using the following MeSH subject headings: (CSF shunts) AND (bacterial infection OR prosthesis-related infection OR catheter-related infection) AND (treatment OR outcome) AND (antibacterial agents OR injections OR antibiotics OR device removal OR ventriculostomy OR combined modality therapy). Searches were limited to studies in patients younger than 18 years of age, the management of initial (not repeat) CSF shunt infection, and to the English language.
Authors performed an updated literature search (in PubMed and Cochrane Central) for this guideline chapter through a medical librarian at the Congress of Neurological Surgeons Guidelines office using the below-mentioned existing search terms to update the original search through November 30, 2019.
Search Results
A total of 342 abstracts were screened and 69 full- text articles were retrieved for review. The details of this process are described in Part 1, the introduction and methodology section of these guidelines.5 An examination of the reference lists of these 69 full-text articles yielded an additional 24 articles that warranted full-text review (Fig. 2). Subsequent review of the full texts of these 93 articles led to the exclusion of 66 articles based on predefined criteria, leaving 27 articles as the basis for the evidentiary tables for this particular recommendation. Reasons for exclusion of full-text articles included the following: literature review (n = 19); no treatment outcomes given (n = 14); pediatric patients not reported separately (n = 6); wrong target population (n = 1); small sample size (n = 19); not a full report of a clinical study (n = 2); not relevant to the study question (n = 3); and other (n = 2).
An additional 2 studies out of the 62 new studies yielded by the 2020 underwent full text review, but were excluded. No new studies met inclusion criteria from the original guideline. (Fig 3)
Results
In general, the methodological quality of the evidence related to this recommendation was poor. The studies that met our inclusion criteria were typically descriptive series of small numbers of patients and were vulnerable to the biases and limitations of a retrospective study design. Because the studies relied on the accuracy and completeness of the medical record, the control of potentially confounding variables was nonexistent. Although most studies did compare outcomes between patient groups treated under alternative management protocols, the rationale behind why a particular treatment was assigned to a particular patient group was not clearly described, leading to significant issues with selection bias. For those studies describing the outcomes of a single management protocol, between-study comparisons of results was hampered by widely disparate management protocols and the use of nonuniform outcome measures (and definitions thereof). These limitations precluded, for the most part, any meaningful quantitative synthesis of the data; what follows is a largely qualitative review of the evidence relevant to this recommendation.
Despite the overall predominance of Class III data, the 13 studies presented in evidentiary Tables 110-16 and 217-22 are quite suggestive of the notion that in the management of CSF shunt infection, supplementation of antibiotic treatment with partial (externalization) or complete shunt hardware removal should be considered. Two Class II studies provide particularly compelling evidence in favor of a combined medical and surgical management of CSF shunt infection, and deserve to be elaborated on further.
In 1980, James et al19 published the results of a moderate-quality randomized controlled trial in which 10 patients with evidence of CSF shunt infection were randomized to each of 3 different treatment arms: 1) complete shunt removal, systemic antibiotics, and either external ventricular drainage or ventricular taps for decompression and intrathecal antibiotic administration, with delayed shunt replacement; 2) complete shunt removal and immediate shunt replacement with intrashunt and systemic antibiotics; or 3) intrashunt and systemic antibiotics without shunt removal. The outcome was negative ventricular CSF cultures 48 hours after cessation of antibiotic therapy and again within 4 months of completion of therapy. All 10 patients who underwent complete shunt removal, systemic antibiotics, and either external ventricular drainage or ventricular taps for decompression and intrathecal antibiotic administration were successfully treated. Nine of 10 patients treated with complete shunt removal and immediate shunt replacement with intrashunt and systemic antibiotics achieved therapeutic trast, only 3 of 10 patients who received systemic and intrathecal antibiotics without shunt removal were successfully treated. The treatment results in this latter group rather clearly demonstrate that shunt removal, rather than antibiotic therapy (including intrathecal therapy), was responsible for the improved outcomes seen in the comparison groups. Secondary outcomes also were consistent with a benefit toward surgical removal of the shunt, as length of hospital stay was lowest in those patients who underwent complete shunt removal with delayed shunt re placement after a course of systemic and intrathecal antibiotics. The only deaths occurred in those patients who received medical management alone. Because of the convincing inferiority of medical management alone, further randomization to this group was halted, but the study was continued as a prospective nonrandomized comparison of treatment outcomes between those patients receiving intrathecal and systemic antibiotics in conjunction with complete shunt removal and delayed versus immediate shunt replacement.18 The principal conclusions remain unchanged.
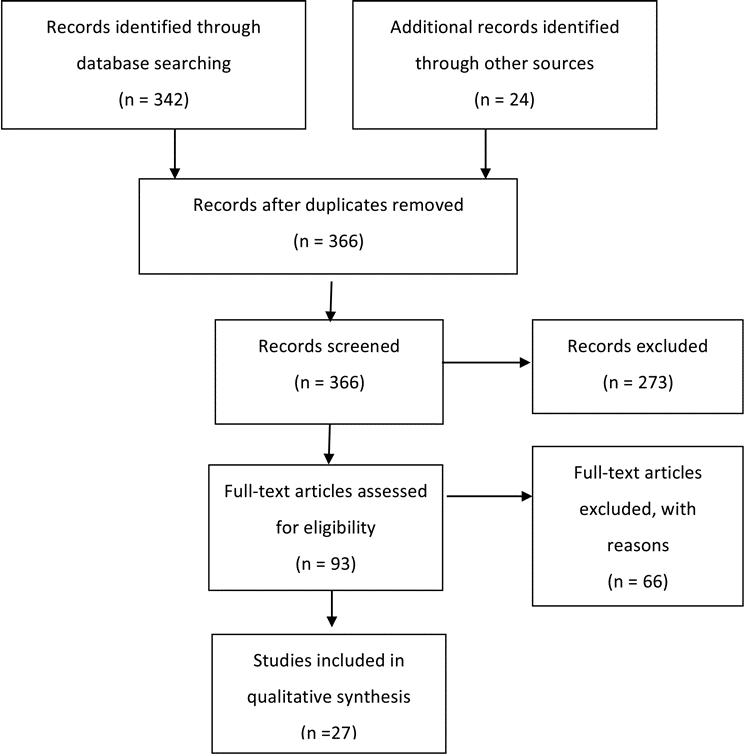
Fig. 2a. Flowchart showing the process involved in identifying relevant literature
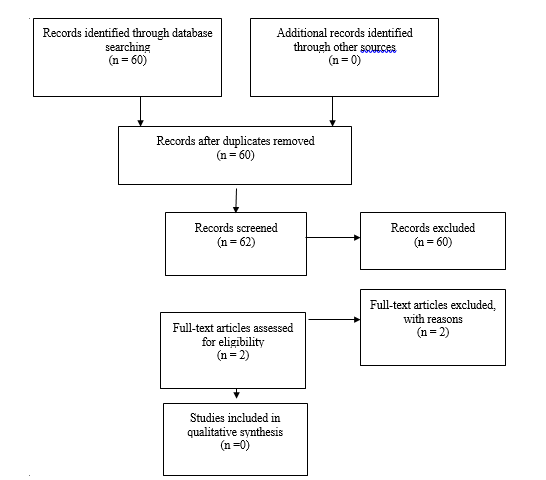
Fig. 2b. Flowchart showing the process involved in identifying relevant literature for the 2020 Update. The criteria for “records excluded” and “full text articles excluded with reasons” are detailed in Part 1 of the Guidelines.
The nearly equivalent treatment outcomes of shunt removal followed by immediate shunt replacement (that is, shunt replacement in infected CSF) versus delayed shunt replacement (that is, shunt replacement after the CSF has been sterilized) in the aforementioned studies by James and colleagues18,19 was suggestive of the potential utility of intrathecal antibiotics in those clinical circumstances in which the shunt must be removed and immediately replaced. As such, these studies provide some evidence applicable to the intrathecal antibiotic recommendation as well. As outlined earlier, it appears that most of the treatment effect comes from shunt removal, making the relative contribution of intrathecal antibiotics to improved outcomes in this scenario rather uncertain. Hence, elevating the recommendation for intrathecal antibiotics to a Level II recommendation, based on these relatively high quality data alone, appears unwarranted.
Additional evidence pertaining to the intrathecal antibiotic recommendation comes largely from Class III studies that examined the results of treatment of ventricular shunt infection in those clinical circumstances in which the infected shunt components are not removed (Table 2)17-22 or only partially removed (that is, externalized) (Table 3)23-26. There was a Class III study that documented a fairly large proportion of patients who achieved therapeutic success—comparable to the success seen in patients who underwent shunt removal—when the patients were treated with intrathecal antibiotics but their shunts were left in situ.16 In addition, Bayston and Rickwood17 documented eradication of staphylococcal VA or VP shunt infection in 5 of 43 patients who underwent antibiotic treatment alone; 4 of the 5 patients who were successfully treated received intrathecal antibiotics. In cases in which ventricular shunt infection was treated with systemic and intrathecal antibiotics along with shunt externalization, either because of the complexity of the shunt infection scenario (for example, multiloculated hydrocephalus) or surgeon preference, a prospective non-randomized study by James and Bradley24 and a Class III study by Arnell et al23 were both able to demonstrate positive treatment outcomes in all patients in their respective case series. Finally, another retrospective case series by James and Bradley27 showed convincingly high cure rates with a significantly shorter length of stay in those patients with an uncomplicated shunt infection (that is, a single shunt system) treated with complete shunt removal together with systemic and intrathecal antibiotics (Table 4). Unfortunately, the absence of a concurrent control group treated with shunt removal and systemic antibiotics alone in this and other studies listed in Table 4 limits the impact of these data to the overall body of evidence.
When examining the studies presented in evidence in Table 323-26 and Table 427-36 it is difficult to say with any degree of clinical certainty whether complete shunt removal leads to better shunt infection treatment outcomes than partial shunt removal. This is due, in part, to the paucity of outcome data comparing the 2 treatment options within the same study population, but also to the confounding effect of intrathecal antibiotic therapy, as described above.
After a full-text review of the contents of papers that were initially identified through our search strategy or our scrutiny of reference lists, predefined criteria led to the exclusion of multiple studies from the evidentiary tables. The recommendations provided above are not materially changed by the exclusion of these studies.
2020 Update
After examining these additional abstracts, two full text articles were assessed for their potential inclusion into the evidence tables. Yakut et al37 was excluded because there was non-consecutive enrollment of patients, and a study by von der Brelie et al38 did not meet inclusion criteria as pediatric patients represented less than 80% of the overall cohort that was reported.
Conclusions
Recommendation: Supplementation of antibiotic treatment with partial (externalization) or with complete shunt hardware removal is an option in the management of CSF shunt infection. Strength of Recommendation: Level II, moderate degree of clinical certainty.
Recommendation: There is insufficient evidence to recommend either shunt externalization or complete shunt removal as a preferred surgical strategy for the management of CSF shunt infection. Therefore, clinical judgment is required. Strength of Recommendation: Level III, unclear degree of clinical certainty.
Recommendation: There is insufficient evidence to recommend the combination of intrathecal and systemic antibiotics for patients with CSF shunt infection in whom the infected shunt hardware cannot be fully removed or must be removed and immediately replaced, or when the CSF shunt infection is caused by specific organisms. The potential neurotoxicity of intrathecal antibiotic therapy may limit its routine use. Strength of Recommendation: Level III, unclear degree of clinical certainty.
It appears that the optimal management of CSF shunt infection requires a multimodality approach. Re- view and critical appraisal of the available evidence regarding the management of ventricular shunt infection allow for a recommendation for the supplementation of antibiotic treatment with partial (externalization) or complete shunt hardware removal with a moderate degree of clinical certainty. However, a recommendation regarding whether complete shunt removal is favored over partial shunt removal (that is, externalization) cannot be made, owing to severe methodological deficiencies in the existing literature. Furthermore, there is insufficient evidence to recommend the use of intrathecal antibiotic therapy as an adjunct to systemic antibiotic therapy in the management of routine CSF shunt infections, or in other clinical scenarios, such as when an infected CSF shunt cannot be completely removed, must be removed and immediately replaced in the face of ongoing CSF infection, or in the setting of ventricular shunt infection caused by specific organisms (for example, gram-negative bacteria).
Deficiencies in the existing literature regarding the management of CSF shunt infection provide a strong rationale for further prospective research into the subject. Key questions that remain unanswered include, but are certainly not limited to the following:
- Defining the optimal duration of antibiotic therapy in the management of CSF shunt infection, with the aim of simultaneously maximizing the probability of successful treatment without reinfection or relapse, and minimizing the length of hospital stay and over-all cost to the health care system
- Refining the indications for intrathecal antibiotic therapy and ascertaining the risk/benefit profile of such therapy (potential adverse effects vs potential reduction in relapse/reinfection rates and shorter hospital stays).
- Definition and validation of standardized treatment outcome measures, based on microbiological or other biomarker-based criteria. This would not only facilitate a comparison of results across studies, but also potentially yield objective criteria that facilitate decision making in other contentious areas of CSF shunt infection management, such as the optimal timing of shunt reimplantation.
Perhaps the best strategy to treat ventricular shunt infection is to continue our focus on the prevention of this significant complication of CSF shunt therapy.
Based on the assessments made in the 2020 update, the authors concluded that no new literature exists to support any change or revision to the current guideline.
Acknowledgments
We acknowledge the American Association of Neurological Surgeons (AANS)/Congress of Neurological Surgeons (CNS) Joint Guidelines Committee for the members’ reviews, comments, and suggestions; Laura Mitchell, Guidelines Project Manager for the CNS, for her contributions; Pamela Shaw, research librarian, for her assistance with the literature searches; Kevin Boyer for his assistance with data analysis; and Sue Ann Kawecki for her assistance with editing. We also acknowledge the following peer reviewers for their contributions to review the update to the guidelines: Jennifer Sweet, MD, Brandon Rocque, MD, Christoph Greissenauer, MD, Jeffrey Olson, MD.
Disclosure
The systematic review and evidence-based guidelines were funded exclusively by the CNS and AANS Pediatric Section, which received no funding from outside commercial sources to support the development of this document.
Conflict(s) of Interest: None. All members of the Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Task Force declared any potential conflicts of interest prior to beginning work on this evidence review.
Conflict(s) of Interest: None. All Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Update Task Force members declared any potential conflicts of interest prior to beginning work on this systematic review and evidence-based guidelines.
Author contributions to the study and manuscript preparation include the following. Conception and design: AANS/CNS Joint Section on Pediatrics. Acquisition of data: all authors. Analysis and interpretation of data: all authors. Drafting the article: Tamber. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Flannery. Administrative/technical/material support: all authors. Study supervision: Flannery.
Evidence Tables
| First Author & Year |
Study Description |
Data Class, Quality and Reasons |
Results and Conclusions |
|
Forrest et al., 1987
|
12 shunt infections with positive blood cultures (but sterile CSF) treated with IV antibiotics and complete shunt removal with immediate shunt replacement.
Outcome = no evidence of recolonization at last follow-up (3-16 years)
|
Class III
Retrospective case series
11 patients with positive blood and CSF cultures were treated with IV antibiotics, shunt removal and EVD with delayed shunt replacement, but their treatment outcomes are not presented
|
12/12 patients with positive blood cultures but sterile CSF are without evidence of recolonization at last follow-up
Difficult to interpret these findings in isolation |
|
Odio et al., 1984
|
59 shunt infections managed with systemic antibiotics alone (A. n=13); systemic antibiotics + immediate shunt removal (B. n=37); or systemic antibiotics + delayed shunt removal (C. n=9).
Outcome = cure (absence of shunt re-infection or relapse) |
Class III
Retrospective case series
Poor control of confounders
Reasons for immediate vs. delayed shunt removal not given (selection bias)
Timing of outcome assessment not given
|
Cure in 8/13 patients treated with antibiotics alone, 34/37 patients treated with antibiotics + immediate shunt removal, and 8/9 patients with antibiotics + delayed shunt removal
Results suggest a poorer outcome without shunt removal |
|
|
Walters et al., 1984
|
267 infections treated in 222 patients. 92 treated medically (85 systemic, 7 systemic + IT); 117 treated medical + surgical (21 shunt removed and immediately replaced under antibiotic coverage, 51 shunt removal + antibiotics + delayed shunt replacement, 20 shunt removal + EVD/shunt externalization + IT antibiotics, 25 shunt removal + antibiotics without shunt replacement); 58 no specific treatment of shunt infection (e.g. unrecognized shunt infection).
Outcome = death
Outcome = death
|
Class III
Retrospective case series
Definition of cure (another tabulated outcome) not defined
Multiple different permutations and combinations of treatment without clear criteria matching a particular patient with a particular treatment (selection bias)
Nontraditional outcome
|
37% mortality with medical management alone
18% mortality with medical + surgical therapy; lowest mortality in those who had shunt removed and replaced under antibiotic coverage in a single operation
Addition of surgical therapy appears to lower morbidity and mortality of shunt infection |
|
|
Schoenbaum et al., 1975
|
98 shunt infections over 442 shunt procedures in 289 patients. Initial treatment based on shunt type – VP shunts were all completely removed and treated with IV antibiotics; some patients with VA and V-ureteral shunts were initially managed with IV antibiotics alone.
Outcome = death
|
Class III
Retrospective case series
Poor control of confounders
Selection bias
Also cite “control of infection” as outcome, but not clearly defined
Nontraditional outcome
|
1/30 patients treated with complete shunt removal and IV antibiotics died; 28/30 had control of infection
6/43 patients with VA and V-ureteral shunts treated with IV antibiotics alone died; 13/43 had control of infection
Shunt removal is required for improved outcome |
|
Shurtleff et al., 1974
|
67 patients with shunt infection treated with IV antibiotics alone (A. n=22); IV antibiotics + shunt revision (B. n=14); IV antibiotics + complete shunt removal and replacement (C. n=12); IV + IT antibiotics + complete shunt removal and replacement (D. n=7); IV + IT/intra-shunt antibiotics alone (E. n=10); or IV + IT antibiotics with shunt revision (F. n=2).
Outcome = cure (no symptoms and at least 6 negative blood cultures and two negative ventricular/shunt CSF cultures during a 6 month period off antibiotics)
|
Class III
Retrospective case series
No control of confounders
Rationale for selecting different therapy for different infections not clear (“therapy evolved during the study”) (selection bias)
|
Cure in 2/22 (A), 3/14 (B), 12/12 (C), 7/7 (D), 3/10 (E), 0/2 (F).
Highest cure rate in those who had complete shunt removal and replacement, irrespective of whether or not they received supplemental IT antibiotics
|
|
Morrice et al., 1974
|
Patients with colonization of VA shunt valves treated with antibiotics alone (n=14); removal and immediate replacement of shunt (n=23); or removal of shunt with a period of external drainage, followed by delayed shunt insertion (n=18).
Outcome = “alive and well” at 6 months
|
Class III
Retrospective case series
Poor control of confounders
Selection bias
Route of administration of antibiotics not specified
Unclear if patients who underwent surgical treatment (immediate or delayed shunt replacement) received supplemental antibiotics
No specific microbiological component of outcome
|
2/14 patients treated with antibiotics alone are alive and well at 6 months
11/23 patients treated with removal and immediate replacement of shunt are alive and well at 6 months
8/18 patients treated with removal of shunt with a period of external drainage, followed by delayed shunt insertion are alive and well at 6 months
Results suggest that shunt removal is required to optimize outcome
|
|
Nicholas et al., 1970
|
60 infections of VA shunts treated with IV antibiotics (also IT antibiotics if the CSF was infected) and delayed shunt replacement (n=33 infections) or with IV antibiotics and immediate shunt replacement (n=27 infections).
Outcome = “subsequent good health of the patient and freedom from bacteremia”
|
Class III
Retrospective case series
No control of confounders
Selection bias
Nondescript outcome
|
Successful treatment in 24/33 infections treated with IV antibiotics (also IT antibiotics if the CSF was infected) and delayed shunt replacement vs. 21/27 infections treated with with IV antibiotics and immediate shunt replacement.
Recurrence/relapse higher in those with immediate shunt replacement
|
|
Table 2. Systemic and Intrathecal Antibiotic Treatment with Shunt Left In Situ or Removed and Immediately Replaced
| Author |
Study Description |
Data Class, Quality and Reasons |
Results and Conclusions |
|
James et al., 1981
|
50 patients (30 reported in James 1980 RCT) with shunt infection treated with shunt removal, systemic antibiotics and either EVD or ventricular taps for decompression and antibiotic administration (A: n=22); removal and immediate replacement of shunt with intrashunt and systemic antibiotics (B: n=17); or intrashunt and systemic antibiotics without shunt removal (C: n=11).
Outcome = negative ventricular CSF cultures 48h after cessation of antibiotic therapy and again within 4 months of completion of therapy.
|
Class II
Prospective non-randomized cohort
Continuation of James 1980 RCT – high incidence of failures in medical management arm (i.e. no shunt removal) made further randomization unjustified
|
21/22 patients in group A were successfully treated
15/17 patients in group B were successfully treated
4/11 patients in group C were successfully treated
Suggests better treatment outcomes with shunt removal
Suggests that IT antibiotics may be of use if shunt must be removed and immediately replaced rather than replaced in a delayed fashion (when infection has been cleared) |
|
James et al., 1980
|
30 patients with shunt infection treated with shunt removal, systemic antibiotics and either EVD or ventricular taps for decompression and antibiotic administration (A: n=10); removal and immediate replacement of shunt with intrashunt and systemic antibiotics (B: n=10); or intrashunt and systemic antibiotics without shunt removal (C: n=10).
Outcome = negative ventricular CSF cultures 48h after cessation of antibiotic therapy and again within 4 months of completion of therapy. |
Class II
RCT with design flaws
Suboptimal randomization and allocation
Baseline characteristics of treatment groups not documented
Unclear if outcome assessment blinded
Underpowered (but study terminated early for harm)
|
10/10 patients in group A were successfully treated
9/10 patients in group B were successfully treated
3/10 patients in group C were successfully treated
length of hospital stay lowest in group A
only deaths occurred in group C
Suggests better treatment outcomes with shunt removal
Suggests that IT antibiotics may be of use if shunt must be removed and immediately replaced rather than replaced in a delayed fashion (when infection has been cleared) |
|
Bayston et al., 1981
|
43 children with Staphylococcal VA or VP shunt infection treated with antibiotics alone (systemic or systemic + IT)
Outcome = eradication of infection (response during treatment with no clinical relapse, followed by repeated normal serological and bacteriological studies)
|
Class III
Retrospective case series
Timing of outcome assessment not clear
|
Eradication of infection in 5/43 patients.
4/5 of those patients with eradication of infection received IT antibiotics
No eradication of S. aureus shunt infection
Suggest the utility of supplemental IT antibiotics if the shunt cannot be removed |
|
|
Wald et al., 1980
|
20 patients with shunt infection treated with daily IT antibiotics (with systemic antibiotics) without removal of the shunt or EVD placement.
Outcome = cure (2 or 3 sterile CSF cultures 72h following completion of antibiotics)
|
Class III
Retrospective case series
Pharmacodynamic study
|
“cure” in 5/7 patients receiving at least 7 days of IT methicillin
“cure” in 4/5 patients treated with IT gentamicin
“cure” in 6/7 patients receiving a single 2-week course of IT cephalothin
Rates of “cure” appear higher than medically treated patients receiving systemic antibiotics alone |
|
Sells et al., 1977
|
20 gram negative shunt infections receiving 25 total treatment trials. Treatments were no treatment (n=2); systemic antibiotics alone (n=4); systemic and intraventricular antibiotics alone (n=4); systemic antibiotics plus in situ shunt replacement (i.e. into infected tract) or incomplete shunt replacement (n=2); systemic and intraventricular antibiotics with in situ shunt replacement (i.e. into infected tract) or incomplete shunt replacement (n=4); systemic and intraventricular antibiotics with complete shunt removal or replacement in a new site (n=9).
Outcome = cure (asymptomatic patient with at least 6 negative blood cultures and 2 negative ventricular or shunt CSF cultures obtained during a 6 month period off antibiotics)
|
Class III
Retrospective case series
Poor control of confounders
Selection bias
Very small numbers receiving each individual treatment
|
Cure in 0/2 patients receiving no treatment
Cure in 1/4 patients receiving systemic antibiotics alone
Cure in 0/4 patients receiving systemic and intraventricular antibiotics alone
Cure in 1/2 patients receiving systemic antibiotics plus in situ shunt replacement or incomplete shunt replacement
Cure in 0/4 patients receiving systemic and intraventricular antibiotics with in situ shunt replacement or incomplete shunt replacement
Cure in 9/9 patients receiving systemic and intraventricular antibiotics with complete shunt removal or replacement in a new site
Clear advantage of complete vs. incomplete shunt removal No clear additional advantage of IT antibiotics in those treated medically or medically with in situ shunt replacement or incomplete shunt replacement
|
|
McLaurin et al., 1975
|
25 shunt infections (23 VA and 2 VP) treated with IV + IT antibiotics and delayed shunt replacement (n=4), IV + IT antibiotics with immediate shunt replacement (n=10), or IV + IT antibiotics alone (n=11).
Outcome = absence of residual infection at last follow-up (6mo-5 yr)
|
Class III
Retrospective case series
Characteristics of those patients successfully treated with IV + IT antibiotics alone (i.e. without shunt removal and replacement) not documented (selection bias)
Extension of McLaurin 1973 series
|
Absence of residual infection at last follow-up in all 24 surviving patients (infection believed to have been cured in the one patient who died)
Suggests that IT antibiotics may be of use if shunt is not removed or must be removed and immediately replaced
Shunt removal may not be necessary for successful treatment of shunt infection if IT antibiotics are administered.
|
|
James et al., 2008
|
Prospective nonrandomized study of 2 protocols for treating complicated shunt infections (multiloculated, multi-organism, infection at other site in body).
- n=21 treated with IV (2 weeks) and IT antibiotics injected through EVD (n=10) or reservoir of externalized shunt (n=11) (2x/week for 2 weeks). Three weeks of antibiotics in total.
Outcome = cure (cultures 48h after cessation of antibiotics, at time of new shunt placement, and 3-6 months later remained negative).
- n=18 treated with IV (2 weeks) and IT antibiotics injected through EVD or reservoir of externalized shunt (1x/week for 2 weeks). Three weeks of antibiotics in total.
Outcome = cure (cultures 24h after cessation of antibiotics, at time of new shunt placement, and 3-6 months later remained negative).
|
Class III
Nonrandomized, prospective case series
Outcome is different for each treatment group
|
All patients treated according to either protocol were cured.
LOS protocol A = 25.1d vs protocol B = 19.7d.
No recurrent shunt infections during the follow-up period.
Patients with complicated shunt infections can be successfully treated successfully with 2 weeks of once daily IT therapy concurrent with 3 weeks of IV therapy (and EVD or shunt externalization).
|
|
Arnell et al., 2007
|
Retrospective review of 34 consecutively treated intravenricular shunt infections treated with externalization of the ventricular catheter proximal to the valve, daily IT injections (generally guided by CSF antibiotic concentrations, median 8 days) and IV antibiotics (median 10 days). Usually no antibiotics after shunt replacement.
Outcome = cure (sterilization of CSF and resolution of clinical symptoms).
|
Class III
Retrospective case series
no control of confounders
|
CSF sterilized in 1/3, 7/8, 20/20 and 6/6 cases after 1, 2, 3, and >3 days of therapy (externalization of ventricular catheter and start of IT antibiotics). Clinical symptoms resolved in parallel with the sterilization of CSF.
Despite the ventricular catheter being left in place and the short duration of therapy, the treatment protocol results in quick CSF sterilization, a low relapse rate, and survival of all patients in this series.
|
|
Wang et al., 1999
|
23 patients treated according to a documented management protocol (externalization of distal catheter unless failure to sterilize CSF, empiric followed by tailored antibiotics for 10 days following sterilization of CSF, reimplant shunt if cultures remain negative for 3 days off antibiotics). Comparison group 10 historical controls treated with an undisclosed regimen.
Outcome = recurrence (re-infection with same organism within 6 months).
|
Class III
Comparative study with historical controls
No control of confounders
3 patients had a ventricular reservoir only
Details of treatment of historical control patients not clear (“duration of antibiotic therapy for each individual case was decided arbitrarily”)
|
Reinfection 0/15 patients treated under protocol (8 patients did not require shunt re-insertion) vs. 2/10 treated before protocol
Shorter hospital stay in those treated under the protocol.
Of those treated under the protocol, patients with a “complex” shunt system required longer hospitalization.
This treatment protocol may be effective in the management of shunt infection
|
|
Ronan et al., 1995
|
41 episodes of infection in 39 children treated with antibiotics (28 IV and oral, 11 IV + IT + oral, 4 IT + IV, 1 IT + oral) and surgical treatment (complete or partial shunt removal and immediate or delayed replacement with or without external ventricular drainage).
Outcome = absence of relapse (re-infection with same organism) at 3 months, and was verified by the absence of relapse for the follow-up period (min. 1 year)
|
Class III
Retrospective case series
Selection bias
Overall management approach too varied to allow for reasonable conclusions to be made
|
Absence of relapse in 31, relapse in 6, death in 4 (not directly related to shunt infection).
Outcome not dependent on length of antibiotic treatment or use of IT antibiotics
Surgical approach to treatment too varied to permit conclusions re: efficacy. Complete shunt replacement associated with lower risk of relapse vs. partial replacement, and delayed replacement had better outcomes vs. immediate replacement.
|
|
Kestle et al., 2006
|
70 patients from 10 centers followed prospectively for 1 year following successful treatment of shunt infection. Initial management was shunt externalization and antibiotics in 17; shunt removal, EVD insertion and antibiotics in 50; and antibiotic treatment alone in 3.
Outcome = culture-proven reinfection (same or different organism).
|
Class II
Prospective multicenter observational study
Reinfection rates in those externalized versus completely removed not provided separately
Timing of outcome assessment not clear
|
Reinfection occurred in 18 patients (26%) – 12 were due to the same initial organism and 6 were different organisms.
Reinfection risk was not associated with length of antibiotic treatment.
This study reconfirms the high reinfection rate in patients receiving treatment for shunt infection.
|
|
Shimuzu et al., 2012
|
Retrospective chart review of 36 patients who underwent shunt removal, EVD placement (4 patients had externalization prior to EVD placement), IV antibiotics and eventual shunt replacement compared to 9 patients who underwent shunt removal, IV antibiotics and ETV for treatment of shunt infection.
Outcome = recurrence of CSF infection within 6 months after shunt reinsertion or ETV.
|
Class III
Retrospective case series
no control of confounders
Selection bias
|
Of those treated with shunt removal then reinsertion, 10/36 experienced CSF reinfection
This study reconfirms the high reinfection rate in patients receiving treatment for shunt infection.
|
|
James et al. (2), 2008
|
Retrospective nonrandomized comparison of 2 protocols for treating uncomplicated shunt infections (single shunt system, single organism, noncompartmentalized hydrocephalus). A. n=25 shunt removal/EVD, IV antibiotics until clinical course and CSF values suggested cure of infection, IT antibiotics 2x/week through EVD or at times of ventricular puncture.
Outcome = cure (cultures 48h after cessation of antibiotics, at time of new shunt placement, and 3-6 months later remained negative).
- n=15 shunt removal/EVD, IV antibiotics until clinical course and CSF values suggested cure of infection, IT antibiotics 1x/week through EVD or at times of ventricular puncture.
Outcome = cure (cultures 24h after cessation of antibiotics, at time of new shunt placement, and 3-6 months later remained negative)
|
Class III
Retrospective comparative study
Outcome is different for each treatment group
|
All patients treated according to either protocol were cured.
Duration of IV antibiotics protocol A = 9.7d vs protocol B = 9.9d.
Patients with a single shunt infected with a single organism and with noncompartmentalized hydrocephalus may be successfully treated without a prolonged antibiotic course and lengthy hospital stay, provided the shunt is completely removed.
|
|
Schuhmann et al., 2005
|
35 consecutive culture proven shunt infections were treated with antibiotics, surgery for shunt removal/EVD placement or shunt externalization and eventual reinternalization of the shunt.
Outcome = shunt reinfection.
|
Class III
Prospective case series
Details of management of shunt infection not clear (e.g. number who underwent complete vs. incomplete shunt removal; number who had intrathecal supplementation to systemic antibiotic therapy, if any)
Outcomes not provided separately for those externalized versus completely removed
|
6/33 patients experienced a shunt reinfection.
This study reconfirms the high reinfection rate in patients receiving treatment for shunt infection.
|
|
Turgut et al., 2005
|
37 infections in 35 patients. 31 patients treated with shunt removal, EVD and systemic + IT antibiotics. 4 patients treated with medical management alone.
Outcome = death.
|
Class III
Retrospective case series
Nontraditional outcome
Criteria for treating 4 patients medically not clear (selection bias)
|
2/31 patients treated with shunt removal, EVD and systemic + IT antibiotics died
1/4 patients treated medically died
good outcomes with IT therapy, but no patients underwent shunt removal with systemic antibiotics alone
|
|
Mancao at al., 1998
|
29 consecutive shunt infections treated. 27 patients had shunt removal +/- external drainage. All had IV antibiotics, and 6 had supplemental IT antibiotics.
Outcome = relapse of infection.
|
Class III
Retrospective case series
Definition of outcome (relapse) not provided, nor was follow-up period defined
Criteria for IT antibiotics not given
|
27 patients had successful treatment (no relapse). 2 deaths not clearly related to shunt infection
This study demonstrates a lower rate of reinfection than other studies, but the data is of poor quality
|
|
Stamos et al., 1993
|
23 consecutive gram negative shunt infections, managed with complete shunt removal and EVD, and IV antibiotics (n=19) or IT antibiotics (n=2, for persistent positive cultures).
Outcome = “cure” (asymptomatic and at least 3 negative cultures off antibiotics), after which shunt was reinserted
|
Class III
Retrospective case series
No control of confounders
|
All patients achieved cure with shunt removal, EVD placement, and antibiotics (19 IV, 2 IV + IT)
On late follow-up of 19 patients (>6 months), 4 had subsequent coag. neg. Staph. Infection
Despite initial success, reinfection rates appear similarly high when compared to other studies
|
|
Kontny et al., 1993
|
28 infections in 25 patients, managed with IV antibiotics and immediate removal of the shunt system (n=24) or IV antibiotics alone (n=4).
Outcome = re-infection or relapse within 1 month of completion of therapy.
|
Class III
Retrospective case series
Details of management of infection not given
Short follow-up
|
All patients were without re-infection or relapse
Short follow-up precludes definitive conclusions
|
|
James et al., 1984
|
18 infections (13 following initial shunt procedure and 5 following revisions) in low birth weight infants (<2000g) treated promptly with shunt removal and IV + IT antibiotics (see James 1980).
Outcome = cure
|
Class III
Retrospective case series
Outcome not clearly defined (? Negative cultures 48h after cessation of antibiotics and within 4 months of completion of therapy, as per James 1980)
|
All except one patient demonstrated cure when treated according to protocol
good outcomes with IT therapy, but no patients underwent shunt removal with systemic antibiotics alone
|
|
Scarff et al., 1978
|
57 children with shunt-related ventricular infection treated with IV + IT antibiotics and external ventricular drainage (either shunt removal and EVD, or shunt externalization).
Outcome = clearance of infection (3 consecutive cultures with negative growth at 48h)
|
Class III
Retrospective case series
Number of patients receiving each surgical therapy (shunt removal and EVD, or shunt externalization) not documented, nor were their outcomes differentially reported
|
54/57 patients demonstrated clearance of infection
good outcomes with IT therapy, but no patients underwent shunt removal with systemic antibiotics alone
|
|
References
- Simon TD, Riva-Cambrin J, Srivastava R, Bratton SL, Dean JM, Kestle JR. Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. Journal of neurosurgery Pediatrics. 2008;1(2):131-137.
- Simon TD, Hall M, Riva-Cambrin J, et al. Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article. Journal of neurosurgery Pediatrics. 2009;4(2):156-165.
- Whitehead WE, Kestle JR. The treatment of cerebrospinal fluid shunt infections. Results from a practice survey of the American Society of Pediatric Neurosurgeons. Pediatric neurosurgery. 2001;35(4):205-210.
- Schreffler RT, Schreffler AJ, Wittler RR. Treatment of cerebrospinal fluid shunt infections: a decision analysis. The Pediatric infectious disease journal. 2002;21(7):632-636.
- Anderson EJ, Yogev R. A rational approach to the management of ventricular shunt infections. The Pediatric infectious disease journal. 2005;24(6):557-558.
- Fan-Havard P, Nahata MC. Treatment and prevention of infections of cerebrospinal fluid shunts. Clinical Pharmacy. 1987;6(11):866-880.
- Gutierrez-Gonzalez R, Boto GR, Perez-Zamarron A. Cerebrospinal fluid diversion devices and infection. A comprehensive review. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2012;31(6):889-897.
- Treatment of infections associated with shunting for hydrocephalus. Working Party on the Use of Antibiotics in Neurosurgery of the British Society for Antimicrobial Chemotherapy. Br J Hosp Med. 1995;53(8):368-373.
- Yogev R. Cerebrospinal fluid shunt infections: a personal view. Pediatric Infectious Disease. 1985;4(2):113-118.
- Forrest DM, Tabara ZB, Towu E, Said AJ. Management of the colonised shunt. Z Kinderchir. 1987;42 Suppl 1:21-22.
- Odio C, Mohs E, Sklar FH, Nelson JD, McCracken GH, Jr. Adverse reactions to vancomycin used as prophylaxis for CSF shunt procedures. Am J Dis Child. 1984;138(1):17-19.
- Schoenbaum SC, Gardner P, Shillito J. Infections of cerebrospinal fluid shunts: epidemiology, clinical manifestations, and therapy. J Infect Dis. 1975;131(5):543-552.
- Walters BC, Hoffman HJ, Hendrick EB, Humphreys RP. Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. Journal of neurosurgery. 1984;60(5):1014-1021.
- Morrice JJ, Young DG. Bacterial colonisation of Holter valves: a ten-year survey. Dev Med Child Neurol. 1974;16(6 Suppl 32):85-90.
- Nicholas JL, Kamal IM, Eckstein HB. Immediate shunt replacement in the treatment of bacterial colonisation of Holter valves. Dev Med Child Neurol Suppl. 1970;22:Suppl 22:110+.
- Shurtleff DB, Foltz EL, Weeks RD, Loeser J. Therapy of staphylococcus epidermidis: infections associated with cerebrospinal fluid shunts. Pediatrics. 1974;53(1):55-62.
- Bayston R, Rickwood AM. Factors involved in the antibiotic treatment of cerebrospinal fluid shunt infections. Zeitschrift fur Kinderchirurgie : organ der Deutschen, der Schweizerischen und der Osterreichischen Gesellschaft fur Kinderchirurgie = Surgery in infancy and childhood. 1981;34(4):339-345.
- James HE, Walsh JW, Wilson HD, Connor JD. The management of cerebrospinal fluid shunt infections: a clinical experience. Acta neurochirurgica. 1981;59(3-4):157-166.
- James HE, Walsh JW, Wilson HD, Connor JD, Bean JR, Tibbs PA. Prospective randomized study of therapy in cerebrospinal fluid shunt infection. Neurosurgery. 1980;7(5):459-463.
- McLaurin RL. Treatment of infected ventricular shunts. Child's brain. 1975;1(5):306-310.
- Sells CJ, Shurtleff DB, Loeser JD. Gram-negative cerebrospinal fluid shunt-associated infections. Pediatrics. 1977;59(4):614-618.
- Wald SL, McLaurin RL. Cerebrospinal fluid antibiotic levels during treatment of shunt infections. Journal of neurosurgery. 1980;52(1):41-46.
- Arnell K, Enblad P, Wester T, Sjolin J. Treatment of cerebrospinal fluid shunt infections in children using systemic and intraventricular antibiotic therapy in combination with externalization of the ventricular catheter: efficacy in 34 consecutively treated infections. Journal of neurosurgery. 2007;107(3 Suppl):213-219.
- James HE, Bradley JS. Management of complicated shunt infections: a clinical report. Journal of neurosurgery Pediatrics. 2008;1(3):223-228.
- Ronan A, Hogg GG, Klug GL. Cerebrospinal fluid shunt infections in children. The Pediatric infectious disease journal. 1995;14(9):782-786.
- Wang KC, Lee HJ, Sung JN, Cho BK. Cerebrospinal fluid shunt infection in children: efficiency of management protocol, rate of persistent shunt colonization, and significance of 'off-antibiotics' trial. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1999;15(1):38-43; discussion 43-34.
- James HE, Bradley JS. Aggressive management of shunt infection: combined intravenous and intraventricular antibiotic therapy for twelve or less days. Pediatric neurosurgery. 2008;44(2):104-111.
- Kontny U, Hofling B, Gutjahr P, Voth D, Schwarz M, Schmitt HJ. CSF shunt infections in children. Infection. 1993;21(2):89-92.
- Shimizu T, Luciano MG, Fukuhara T. Role of endoscopic third ventriculostomy at infected cerebrospinal fluid shunt removal. Journal of neurosurgery Pediatrics. 2012;9(3):320-326.
- James HE, Bejar R, Gluck L, et al. Ventriculoperitoneal shunts in high risk newborns weighing under 2000 grams: a clinical report. Neurosurgery. 1984;15(2):198-202.
- Kestle JR, Garton HJ, Whitehead WE, et al. Management of shunt infections: a multicenter pilot study. Journal of neurosurgery. 2006;105(3 Suppl):177-181.
- Mancao M, Miller C, Cochrane B, Hoff C, Sauter K, Weber E. Cerebrospinal fluid shunt infections in infants and children in Mobile, Alabama. Acta paediatrica (Oslo, Norway : 1992). 1998;87(6):667-670.
- Scarff TB, Nelson PB, Reigel DH. External drainage for ventricular infection following cerebrospinal fluid shunts. Child's brain. 1978;4(3):129-136.
- Schuhmann MU, Ostrowski KR, Draper EJ, et al. The value of C-reactive protein in the management of shunt infections. Journal of neurosurgery. 2005;103(3 Suppl):223-230.
- Stamos JK, Kaufman BA, Yogev R. Ventriculoperitoneal shunt infections with gram-negative bacteria. Neurosurgery. 1993;33(5):858-862.
- Turgut M, Alabaz D, Erbey F, et al. Cerebrospinal fluid shunt infections in children. Pediatric neurosurgery. 2005;41(3):131-136.
- Yakut N, Soysal A, Kepenekli Kadayifci E, et al. Ventriculoperitoneal shunt infections and re-infections in children: a multicentre retrospective study. British journal of neurosurgery. 2018;32(2):196-200.
- von der Brelie C, Simon A, Groner A, Molitor E, Simon M. Evaluation of an institutional guideline for the treatment of cerebrospinal fluid shunt-associated infections. Acta neurochirurgica. 2012;154(9):1691-1697.