Guidelines on the Evaluation and Treatment of Patients with Thoracolumbar Spine Trauma
7. Prophylaxis and Treatment of Thromboembolic Events
download pdf Neurosurgery, 2018
Sponsored by: Congress of Neurological Surgeons and the Section on Disorders of the Spine and Peripheral Nerves in collaboration with the Section on Neurotrauma and Critical Care
Endorsed by: The Congress of Neurological Surgeons (CNS) and the American Association of Neurological Surgeons (AANS)
P. B. Raksin, MD,1 James S. Harrop, MD,2 Paul A. Anderson, MD,3 Paul M. Arnold, MD,4 John H. Chi, MD, MPH,5 Andrew T. Dailey, MD,6 Sanjay S. Dhall, MD,7 Kurt M. Eichholz, MD,8 Daniel J. Hoh, MD,9 Sheeraz Qureshi, MD, MBA,10 Craig H. Rabb, MD,11 Michael G. Kaiser, MD,12 and John E. O’Toole, MD13
1. Division of Neurosurgery, John H. Stroger, Jr. Hospital of Cook County and Department of Neurological Surgery, Rush University Medical Center, Chicago, Illinois
2. Departments of Neurological Surgery and Orthopedic Surgery, Thomas Jefferson University, Philadelphia, Pennsylvania
3. Department of Orthopedics and Rehabilitation, University of Wisconsin, Madison, Wisconsin
4. Department of Neurosurgery, University of Kansas School of Medicine, Kansas City, Kansas
5. Department of Neurosurgery, Harvard Medical School, Brigham and Women’s Hospital, Boston, Massachusetts
6. Department of Neurosurgery, University of Utah, Salt Lake City, Utah
7. Department of Neurological Surgery, University of California, San Francisco, San Francisco, California
8. St. Louis Minimally Invasive Spine Center, St. Louis, Missouri
9. Lillian S. Wells Department of Neurological Surgery, University of Florida, Gainesville, Florida
10. Department of Orthopaedic Surgery, Weill Cornell Medical College, New York, New York
11. Department of Neurosurgery, University of Utah, Salt Lake City, Utah
12. Department of Neurosurgery, Columbia University, New York, New York
13. Department of Neurological Surgery, Rush University Medical Center, Chicago, Illinois
Correspondence:
P. B. Raksin, MD
Chief, Section of Neurotrauma & Neurocritical Care
Director, Neurosurgery ICU
John H. Stroger, Jr. Hospital of Cook County
Division of Neurosurgery
1900 W. Polk St.
Admin. Bldg., Rm. 641
Chicago, IL 60612
Associate Professor
Department of Neurological Surgery
Rush University Medical Center
Email: patricia_raksin@rush.edu
Keywords: deep venous thrombosis, pulmonary embolism, venous thromboembolic event, thoracolumbar spine fracture, spinal cord injury
Abbreviations
DVT – deep venous thrombosis
EPCC – external pneumatic calf compression
LMWH – low molecular weight heparin
PE – pulmonary embolism
SCI – spinal cord injury
UFH – unfractionated heparin
VTE – venous thromboembolism
No part of this article has been published or submitted for publication elsewhere.
ABSTRACT
Background: Venous thromboembolic events (VTEs), in the form of deep venous thrombosis (DVT) or pulmonary embolism (PE), are common in the population of patients with acute spinal cord injury (SCI) and carry a significant risk for morbidity.
Objective: Three questions were posed, each specific to the cohort of patients with thoracic and/or lumbar spine fractures, to determine: 1) does routine screening for DVT prevent PE in this population; 2) is one regimen of DVT prophylaxis superior to others with respect to prevention of PE; and 3) is there a specific treatment regimen for documented VTE that provides fewer complications than other treatments in this population?
Methods: A literature search was conducted to identify articles the were relevant to the questions posed. Abstracts were reviewed for relevance, and full-text articles were selected. Data abstraction was performed for articles meeting inclusion criteria, level of evidence determined, and a recommendation grade established.
Results: A single article was identified meeting inclusion criteria for question 2. This study provides evidence that while the use of external pneumatic calf compression (EPCC) provides some decrement in the incidence of DVT, the combination of mechanical and chemical (aspirin and dipyridamole) prophylaxis provides even greater reduction. No studies met inclusion criteria for questions 1 or 3.
Conclusion: There is insufficient evidence in the specific setting of thoracic and lumbar fractures to provide discrete recommendations pertaining to the questions posited. However, the consensus of the work group—based on published data from pooled (cervical and thoracolumbar) SCI populations—is that the use of thromboprophylaxis is recommended to reduce the risk of VTE events.
RECOMMENDATIONS
|
Questions
|
- Does routine screening for DVT prevent PE (or VTE-associated morbidity and mortality) in patients with thoracic and lumbar fractures?
- For patients with thoracic and lumbar fractures, is one regimen of VTE prophylaxis superior to others with respect to prevention of PE (or VTE-associated morbidity and mortality)?
- Is there a specific treatment regimen for documented VTE that provides fewer complications than other treatments in patients with thoracic and lumbar fractures?
|
|
Recommendations
|
- There is insufficient evidence to recommend for or against routine screening for DVT in preventing PE (or VTE-associated morbidity and mortality) in patients with thoracic and lumbar fractures.
|
|
Strength of Recommendation: Grade Insufficient
|
|
2. There is insufficient evidence to recommend a specific regimen of VTE prophylaxis to prevent PE (or VTE-associated morbidity and mortality) in patients with thoracic and lumbar fractures.
|
|
Strength of Recommendation: Grade Insufficient
|
|
3. There is insufficient evidence to recommend for or against a specific treatment regimen for documented VTE that would provide fewer complications than other treatments in patients with thoracic and lumbar fractures.
|
|
Strength of Recommendation: Grade Insufficient
|
|
4. Based on published data from pooled (cervical and thoracolumbar) SCI populations, the use of thromboprophylaxis is recommended to reduce the risk of VTE events in patients with thoracic and lumbar fractures.
|
|
Consensus Statement by the Workgroup
|
INTRODUCTION
Goals and Rationale
This clinical guideline was created to improve patient care by outlining the appropriate information gathering and decision-making processes involved in the evaluation and treatment of patients with thoracolumbar spine trauma. The surgical management of these patients often takes place under a variety of circumstances and by various clinicians. This guideline was created as an educational tool to guide qualified physicians through a series of diagnostic and treatment decisions to improve the quality and efficiency of care.
Acute traumatic spinal cord injury (SCI) is associated with an increased risk for venous thromboembolic (VTE) complications of deep venous thrombosis (DVT) and pulmonary embolism (PE). When accounting for differences in level of injury, diagnostic modality employed, and surveillance strategy, the overall incidence of VTE events among patients with acute SCI receiving no or suboptimal prophylaxis has been estimated as 4–100%.1-13 Decision-making regarding thromboprophylaxis for these patients is often complex. Many of the same factors, such as immobility, associated long bone or pelvic fractures, posttraumatic inflammation, and the need for surgical intervention(s) that contribute to this vulnerability must also be taken into account when considering potential benefits and harms (particularly bleeding) associated with available therapeutic modalities. Comorbid traumatic brain injury or visceral injury may further confound this calculus.
Interestingly, several studies suggest the highest incidence of VTE events occurs among patients with thoracic segment SCI. Rossi et al14 observed DVT in 13 of 18 (72%) of patients with “lower limb paralysis” secondary to SCI.14 In a case series of 431 patients treated over a 10-year period, Watson6 noted that the incidence of DVT varied from 6–25% per year, with a rate of thrombosis that was greater among thoracic (23%) than lumbar (12%) or cervical (9%) levels of injury. Winemiller et al15 similarly identified a greater risk for VTE in patients with a thoracic level (relative risk 1.81; P = .032). Jones et al16 analyzed all cases (16,420) admitted over an 11-year period in California that were coded as “acute SCI with fracture of the vertebral column” or “SCI without fracture,” noting a significantly higher incidence (P = .009) of VTE in patients with paraplegia (11%) than tetraplegia (7.8%). Complete paraplegia was significantly associated with the development of VTE (odds ratio 1.80; tetraplegia OR 1.0).16 Waring and Karunas5 did not identify a significant difference in PE or DVT rates for paraplegic compared with tetraplegic patients. However, the highest incidence of VTE events was noted in motor complete paraplegia and the lowest in motor incomplete tetraplegia.5 In a study comparing unfractionated heparin (UFH) and low molecular weight heparin (LMWH) prophylaxis regimens, Worley et al17 demonstrated no significant association between the type of prophylaxis and incidence of DVT; however, paraplegia (5/30, 16.7%), compared with tetraplegia (2/60, 3.3%), significantly increased the incidence of VTE (P = .0388).
Studies also suggest that early initiation of prophylaxis and continuation for a period of approximately 3 months postinjury are effective strategies for the prevention of VTE. El Masri and Silver10 published a case series of 102 consecutive SCI patients, 66 of whom received oral anticoagulation (phenindione) to a target international normalized ratio range of 1.8–2.5. Pulmonary emboli were observed only in patients who were inadequately anticoagulated, who were in the first week of therapy, or who arrived >3 days after injury without previous prophylaxis.10 Walsh and Tribe18 reported that while 15 fatal PEs (66 total) occurred in their cohort of 500 SCI patients within the first 3 months after injury, no fatalities beyond that window were attributable to VTE events. The 2005 Jones et al16 retrospective cohort analysis found that 88% of VTE diagnoses were made within the first 3 months after injury. There was a 5.4% cumulative incidence of VTE over that period. Twenty-five surgeons in the Spine Trauma Study Group participated in a live survey session regarding thromboprophylaxis in the setting of acute SCI and reached consensus for a 3-month postinjury pharmacologic prophylaxis window.19 Implementation of these strategies, however, must be weighed against the potential risk for bleeding in patients with spinal cord or canal hematoma, concomitant traumatic brain injury, and/or a need for operative neurosurgical intervention, as well as those with polytrauma.
Numerous published investigations reported the results of individual or multiple combined prophylactic measures. The regimens are sufficiently heterogeneous; therefore, few generalities can be advanced. The studies may be categorized by the type of prophylaxis investigated: mechanical alone, individual pharmacologic agent, comparative pharmacologic agents, and combined pharmacologic/mechanical modalities.
Becker et al20 randomized a small group (n = 15) of patients with acute SCI to a continuous rotating versus standard stationary bed for the first 10 days after injury. One of 10 patients in the treatment arm and 4 of 5 in the control group were diagnosed with DVT by fibrinogen scanning. The same year, Katz et al21 published a small series (n = 10) examining the effect of 60 min of electrical stimulation on fibrinolytic activity. The results of this pilot study suggested that functional electrical stimulation in SCI may augment fibrinolytic activity and increase venous blood flow.
Reported data concerning DVT incidence associated with various dosing regimens for UFH are inconsistent. Casas et al22 reported no VTE events in a group of 18 acute SCI patients receiving UFH 5000–7500 IU twice daily from “the first days” postinjury until transitioned to wheelchair, whereas Gündüz et al8 reported a DVT rate of 53.3% (16/30) for a small cohort (n = 31) of patients receiving a similar regimen (UFH 5000 IU twice daily). Hachen23 reported a lower incidence of DVT among patients receiving UFH twice daily (3/44, 6.8%) as compared with a historical control group receiving oral anticoagulation (17/76, 21%). Frisbie and Sasahara11 identified no significant difference in the incidence of DVT between acute SCI patients receiving twice-daily UFH dosing (1.15, 6.6%) and no prophylaxis (1/17, 5.8%), with an overall DVT incidence more closely aligned with that noted by Hachen.23 Despite an increased frequency dosing of UFH (5000 IU 3 times daily), Kulkarni et al9 observed a DVT/PE incidence of 26% (26/97 patients). Green et al24 randomized 75 consecutive patients with SCI to fixed (5000 IU twice daily) versus adjusted-dose (activated partial thromboplastin time approximately 1.5 times control) heparin. VTE was detected in 9 of 29 (31%) patients on the fixed regimen and 2 of 29 (7%) of those on the adjusted-dose regimen. While no patient who reached the activated partial thromboplastin time target developed thrombosis, 7 experienced bleeding events (none in the fixed-dose group).
Others have considered the role of LMWH in the prevention of VTE events. A study by Slavik et al25 examined the incidence of both DVT/PE and major bleeding in a retrospective cohort of acute SCI (n = 73) and major orthopedic trauma patients. In the first phase of their trial, 63 patients received enoxaparin (30 mg twice daily) and in the second phase, 72 patients received dalteparin (5000 IU daily). There was no significant difference in the incidence of DVT or PE (1/63, 1.6% enoxaparin group; 7/72, 9.7% dalteparin group). Dalteparin was associated with an absolute risk increase of 8.1%. The risk of major bleeding was similar in both groups (6.4% and 6.9%, respectively). Chiou-Tan et al26 randomized 95 patients with acute SCI to receive either enoxaparin (30 mg twice daily) or dalteparin (5000 IU daily) for 3 months (if motor complete, 2 months if incomplete). They observed similar DVT incidence (3/50, 6% enoxaparin; 2/45, 4% dalteparin) and bleeding rates (1/50, 2% for enoxaparin; 2/45, 4% for dalteparin) for both regimens.
Worley et al17 retrospectively examined 2 thromboprophylaxis regimens adopted at their institution over time: UFH (5000 IU twice daily) and subsequently, dalteparin (5000 IU daily). No significant difference was identified between regimens. However, the incidence of DVT was significantly increased among those patients with para- as opposed to tetraplegia. Similarly, Thumbikat et al27 retrospectively reviewed 2 cohorts of acute SCI patients receiving prophylaxis regimens distinguished by admission date. The first historical group received warfarin plus UFH (5000 IU twice daily), titrated until the international normalized ratio was >2. The latter group received enoxaparin at either a 20- or 40-mg dose. The incidence of DVT was significantly greater in the enoxaparin group (13/72, 18%) than the warfarin plus UFH group (4/101, 4%) and greater in the lower (10/40, 25%) than the higher-dose (3/32, 9.4%) LMWH group. It is important to note that 6 of 13 documented VTE events occurred after stopping anticoagulation. Also, the duration of anticoagulation was not standardized. Bleeding occurred in 8 of 101 patients in the warfarin plus UFH group versus 3 of 72 in the LMWH group. Green et al28 randomized 41 patients to either UFH (5000 IU 3 times daily) or logiparin (3500 antiXa U daily) for a total of 8 weeks. Seven of 21 (34.7%) in the UFH group experienced bleeding or thrombosis, whereas none in the LMWH (0/20) group did (P = .006). Five patients in the UFH group experienced PE (P = .02). Two in the UFH group had bleeding with prolonged activated partial thromboplastin time. The trial was terminated because of a statistically significant increase in the event rate for the UFH group and 2 fatal PEs.
Other groups have advanced the hypothesis that combination therapy—most commonly with sequential compression devices (SCDs) plus a pharmacologic agent—would provide greater benefit than either modality in isolation. In 2003, Spinal Cord Injury Thromboprophylaxis Investigators published a multicenter randomized, controlled trial in which 476 acute SCI patients were randomized to receive UFH (5000 IU 3 times daily) plus SCDs or LMWH (enoxaparin 30 mg 2 times daily) alone for 14 days.29 Ultrasound and venography were performed at 14 days. The overall incidence of VTE was comparable for the 2 regimens (31/49, 63.3% UFH-SCD vs. 38/58, 65.5% LMWH); the enoxaparin group demonstrated a significantly lower incidence of PE. Major bleeding was not significantly different between groups (13/246, 5.3% UFH-SCD vs. 6/230, 2.6% enoxaparin). The same group published a follow-up study encompassing those patients who completed the acute-phase trial without objective evidence of DVT.30 The remaining patients continued to receive either UFH or enoxaparin for the ensuing 6 weeks. An ultrasound was performed at the conclusion of the study period. The overall incidence of VTE neared statistical significance (P = .052) for the benefit of enoxaparin (5/59, 8.5%) over UFH (13/60, 21.7%). Neither regimen demonstrated a statistically significant benefit with respect to DVT or PE incidence. Major bleeding occurred in 1 of 86 (1.2%) UFH patients and no enoxaparin patients.
Maxwell et al31 conducted a retrospective trauma registry review (n = 8269) to identify patients with acute SCI (n = 111). In the first 2 years, patients received UFH (5000 IU twice daily) plus SCDs. In the ensuing 2 years, patients received enoxaparin (30 mg twice daily) plus SCDs. There was no significant difference in the incidence of DVT or PE between regimens (or as compared with the group receiving SCDs alone).31 Finally, Merli et al32 randomized acute SCI patients to placebo, UFH (5000 IU 3 times daily), or UFH plus electrical stimulation groups. Daily fibrinogen scanning was performed, as well as venography at the end of the study period if the fibrinogen remained negative. UFH alone (8/16, 50%) performed no better than placebo (8/17, 47%); however, the group receiving electrical stimulation in addition to UFH (1/15, 6.7%) demonstrated a significantly reduced incidence of DVT (P < .05). When data pooled from the other 2 groups were compared with the stimulation group, the level of significance was even greater (P < .008).
The studies outlined above support some general tenets regarding thromboprophylaxis in the setting of acute SCI. They establish that significant risk for VTE events exists in this patient population as a whole—and perhaps to an even greater extent among patients with thoracic segment injury. They suggest that some prophylaxis is better than no prophylaxis and that while one pharmacologic agent may not be demonstrably superior to another, pharmacologic prophylaxis may be better than mechanical measures alone. Combination therapy may offer additional benefit beyond any singular modality. However, these same studies, while integral to any discussion of the indications for and potential benefits of thromboprophylaxis, share a lack of specificity that limits their applicability to the current investigation. Either SCI is equated with cervical segment pathology, injuries are not stratified by segment, or the investigators did not enroll a sufficient number of thoracic and lumbar segment injuries to warrant inclusion for this analysis.
The authors addressed considerations specific to the occurrence of VTE events in the setting of thoracic and lumbar spine fractures. Three questions were posed:
- Does routine screening for DVT prevent PE (or VTE-associated morbidity and mortality) in patients with thoracic and lumbar fractures?
- For patients with thoracic and lumbar fractures, is one regimen of VTE prophylaxis superior to others with respect to prevention of PE (or VTE-associated morbidity and mortality)?
- Is there a specific treatment regimen for documented VTE that provides fewer complications than other treatments in patients with thoracic and lumbar fractures?
Methods
The guidelines task force initiated a systematic review of the literature relevant to the diagnosis and treatment of patients with thoracolumbar trauma. Through objective evaluation of the evidence and transparency in the process of making recommendations, this evidence-based clinical practice guideline was developed for the diagnosis and treatment of adult patients with thoracolumbar injury. These guidelines are developed for educational purposes to assist practitioners in their clinical decision-making processes. Additional information about the methods used in this systematic review is provided in the introduction and methodology chapter.
Literature Search
The task force members identified search terms/parameters, and a medical librarian implemented the literature search, consistent with the literature search protocol (see Appendix I), using the National Library of Medicine PubMed database and the Cochrane Library (which included the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effect, the Cochrane Central Register of Controlled Trials, the Health Technology Assessment Database, and the National Health Service Economic Evaluation Database) for the period from January 1, 1946 to March 31, 2015, using the search strategies provided in Appendix I.
RESULTS
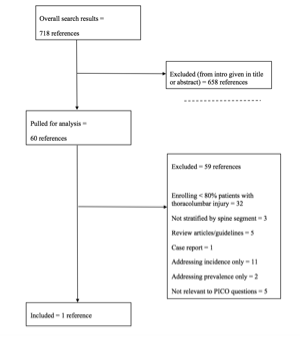
The literature search yielded 697 PubMed results. A separate Cochrane search produced 49 results which, after prescreening for non-English and duplicates, yielded 21 additional references, for a total of 718.
Task force members reviewed all abstracts yielded from the literature search and identified the literature for full text review and extraction, addressing the clinical questions, in accordance with the literature search protocol (Appendix I). Task force members identified the best research evidence available to answer the targeted clinical questions. When level I, II, and/or III literature was available to answer specific questions, the task force did not review level IV studies.
The task force selected 60 articles for full text review. Among these selections, 28 were potentially applicable to question 2, 6 to question 1, and none to question 3. Of the 60 full-text articles, 59 were rejected for not meeting inclusion criteria or for being off topic (most for enrolling <80% patients with thoracolumbar segment injuries or not stratifying results by involved spine segment). One was selected for inclusion in this systematic review (Appendix II).
Inclusion/Exclusion Criteria
Articles were retrieved and included only if they met specific inclusion/exclusion criteria. These criteria were also applied to articles provided by guideline task force members who supplemented the electronic database searches with articles from their own files. To reduce bias, these criteria were specified before conducting the literature searches.
Articles that do not meet the following criteria were, for the purposes of this evidence-based clinical practice guideline, excluded. To be included as evidence in the guideline, an article had to be a report of a study that:
- Investigated patients with thoracolumbar injuries;
- Included patients ≥18 years of age;
- Enrolled ≥80% of thoracolumbar injuries (studies with mixed patient populations were included if they reported results separately for each group/patient population);
- Was a full article report of a clinical study;
- Was not an internal medical records review, meeting abstract, historical article, editorial, letter, or commentary;
- Appeared in a peer-reviewed publication or a registry report;
- Enrolled ≥10 patients per arm per intervention (20 total) for each outcome;
- Included only human subjects;
- Was published in or after 1946 through March 31, 2015;
- Quantitatively presented results;
- Was not an in vitro study;
- Was not a biomechanical study;
- Was not performed on cadavers;
- Was published in English;
- Was not a systematic review, meta-analysis, or guideline developed by others[1];
- Was a case series (therapeutic study) where higher level evidence exists.
Rating Quality of Evidence
The guideline task force used a modified version of the North American Spine Society’s evidence-based guideline development methodology. The North American Spine Society methodology uses standardized levels of evidence (Appendix III) and grades of recommendation (Appendix IV) to assist practitioners in easily understanding the strength of the evidence and recommendations within the guidelines. The levels of evidence range from level I (high quality randomized controlled trial) to level IV (case series). Grades of recommendation indicate the strength of the recommendations made in the guideline based on the quality of the literature. Levels of evidence have specific criteria and are assigned to studies before developing recommendations. Recommendations are then graded based upon the level of evidence. To better understand how levels of evidence inform the grades of recommendation and the standard nomenclature used within the recommendations, see Appendix IV.
Guideline recommendations were written using a standard language that indicates the strength of the recommendation. “A” recommendations indicate a test or intervention is 2 “recommended”; “B” recommendations “suggest” a test or intervention; “C” recommendations indicate a test or intervention or “is an option.” “Insufficient evidence” statements clearly indicate that “there is insufficient evidence to make a recommendation for or against” a test or intervention. Task force consensus statements clearly state that “in the absence of reliable evidence, it is the task force’s opinion that” a test or intervention may be considered. Both the levels of evidence assigned to each study and the grades of each recommendation were arrived at by consensus of the workgroup employing up to three rounds of voting when necessary.
In evaluating studies as to levels of evidence for this guideline, the study design was interpreted as establishing only a potential level of evidence. As an example, a therapeutic study designed as a randomized controlled trial would be considered a potential level I study. The study would then be further analyzed as to how well the study design was implemented and significant shortcomings in the execution of the study would be used to downgrade the levels of evidence for the study’s conclusions (see Appendix V for additional information and criteria).
Revision Plans
In accordance with the Institute of Medicine’s standards for developing clinical practice guidelines and criteria specified by the National Guideline Clearinghouse, the task force will monitor related publications following the release of this document and will revise the entire document and/or specific sections “if new evidence shows that a recommended intervention causes previously unknown substantial harm; that a new intervention is significantly superior to a previously recommended intervention from an efficacy or harms perspective; or that a recommendation can be applied to new populations.”33 In addition, the task force will confirm within 5 years from the date of publication that the content reflects current clinical practice and the available technologies for the evaluation and treatment for patients with thoracolumbar trauma.
DISCUSSION
|
Question 1
|
|
Does routine screening for DVT prevent PE (or VTE-associated morbidity and mortality) in patients with thoracic and lumbar fractures?
|
|
Recommendation 1
|
|
There is insufficient evidence to recommend for or against routine screening for DVT in preventing PE (or VTE-associated morbidity and mortality) in patients with thoracic and lumbar fractures.
|
|
Strength of Recommendation: I
|
|
No relevant articles meeting inclusion criteria were identified for this question.
|
|
Question 2
|
|
For patients with thoracic and lumbar fractures, is one regimen of VTE prophylaxis superior to others with respect to prevention of PE (or VTE-associated morbidity and mortality)?
|
Recommendation 2
|
|
There is insufficient evidence to recommend a specific regimen of VTE prophylaxis to prevent PE (or VTE-associated morbidity and mortality) in patients with thoracic and lumbar fractures.
|
|
Strength of Recommendation: I
|
|
A single study provides level 2 evidence that while EPCC decreases the incidence of DVT, the combination of mechanical and pharmacologic prophylaxis (aspirin and dipyridamole) results in a greater reduction.
|
|
Question 3
|
|
Is there a specific treatment regimen for documented VTE that provides fewer complications than other treatments in patients with thoracic and lumbar fractures?
|
Recommendation 3
|
|
There is insufficient evidence to recommend for or against a specific treatment regimen for documented VTE that would provide fewer complications than other treatments in patients with thoracic and lumbar fractures.
|
|
Strength of Recommendation: I
|
|
No relevant articles meeting inclusion criteria were identified for this question.
|
|
Recommendation 4
|
|
Based on published data from pooled (cervical and thoracolumbar) spinal cord injury populations, the use of thromboprophylaxis is recommended to reduce the risk of VTE events in patients with thoracic and lumbar fractures.
|
|
Strength of Recommendation: Consensus Statement by the Workgroup
|
Ultimately, only 1 article was identified that met inclusion criteria for any of the 3 questions posed. This study provides level II evidence applicable to question 2. Green et al34 published a prospective study comparing mechanical and combined pharmacologic/mechanical regimens for the prevention of DVT in patients with SCI. Twenty-eight consecutive patients with “lower limb paralysis” were randomized to receive either EPCC or EPCC in combination with aspirin (300 mg twice daily) and dipyridamole (75 mg 3 times daily) for the first 30 days postinjury. Daily fibrinogen scanning and every-third-day impedance plethysmography were performed. Positive results were confirmed by venography. Overall, DVT was detected in 9 of 27 (33%) patients analyzed (1 was lost to transfer). This was significant as compared with the 78% DVT rate observed previously in a cohort of 37 patients who received no prophylaxis. The use of EPCC lowered the rate to 40%, while the addition of aspirin and dipyridamole lowered the rate further to 25%. This study was downgraded from level I to level II in recognition of multiple deficiencies: method of randomization not reported, lack of blinding, no power analysis, inadequate reporting of baseline data, no posttreatment assessment, and a discussion referencing untreated “controls” from a previously published study. This now 34-year-old publication reports on a pharmacologic regimen that would be considered “historical” in 2016. Still, the suggestion that combined pharmacologic and mechanical prophylaxis might provide a benefit over mechanical alone is consistent with available literature for the broader topic of “acute spinal cord injury.”
The absence of sufficient evidence to permit discrete recommendations should not be construed as an indication to forego screening or prophylaxis for this acknowledged high-risk group. Rather, this conclusion merely reflects strict adherence to methodology. The literature search strategy for this topic was designed to restrict results to the specific subpopulation of patients with injury to the thoracic or lumbar segments. Most published studies on the topic of VTE prophylaxis in the setting of SCI fail either to distinguish between patients presenting with tetraplegia or paraplegia or to stratify injury by spine segment. The great majority of potentially relevant articles were excluded for failure to reach 80% thoracolumbar injury threshold alone. If a wider net is cast to encompass “acute SCI” as a general subject term, there exists ample evidence, predominantly level III, but with some level I and II studies, for the use of DVT prophylaxis. (Many of these studies were reviewed as scientific foundation in the Introduction section.)
The current American College of Chest Physicians (ACCP) evidence-based clinical practice guideline for Prevention of VTE in Nonorthopedic Surgical Patients recommends the use of dual mechanical and pharmacologic prophylaxis with either low-dose UFH or LMWH for patients with acute SCI (all grade 2C, which is a weak recommendation, based on low or very low quality of evidence).35 The authors assert that for patients at high risk for VTE and average risk for bleeding complications, there is low-quality evidence to suggest that the number of nonfatal VTE events prevented with pharmacologic prophylaxis may exceed (by 10-fold) any nonfatal bleeding complications precipitated. The risk:benefit ratio for patients with high risk for major bleeding, however, is less favorable. In settings where the use of pharmacologic prophylaxis might be contraindicated, the authors recommend the use of mechanical prophylaxis (preferably pneumatic compression stockings) over no prophylaxis, as well as reassessment for the addition of pharmacologic prophylaxis when medically feasible (both grade 2C).
The second edition of Guidelines for the Management of Cervical Spine and Spinal Cord Injuries offers more specific guidance regarding prophylaxis for VTE.36 A level I recommendation is provided for the use of prophylaxis in patients with motor deficit caused by SCI. Additional level I recommendations suggest prophylaxis strategies of: 1) low dose heparin—in combination with pneumatic compression stockings or electrical stimulation, or 2) LMWH, rotating beds, or a combination of modalities. The use of low-dose heparin alone or oral anticoagulation is not recommended (level II). Early initiation (within 72 h) and a 3-month duration for prophylaxis are recommended (level II). The selective use of inferior vena cava filters is recommended for patients who either fail anticoagulation or are not candidates for pharmacologic or other mechanical modalities (level III).
Future Research
The absence of sufficient evidence to offer discrete recommendations regarding VTE prophylaxis for the specific population of patients presenting with thoracic and lumbar spine injuries points to an obvious gap in data. There is evidence available for the larger population of patients with “spinal cord injury.” However, much of this body of literature consists of low quality evidence. Sample sizes are often small. Studies are underpowered to demonstrate effect. Populations may be heterogeneous and often are not stratified by segment of injury. Treatment modalities are not comparable by dosing regimen or duration across studies. Protocols for VTE surveillance and diagnosis vary. Documentation of complications is inconsistent, particularly with respect to bleeding severity.
Given that several studies suggest an increased incidence of VTE events among patients with thoracic segment injury, it would be desirable to better understand not only the pathophysiologic reason for this observation but also how best to provide prophylaxis for this particularly high-risk group of patients. Comparative effectiveness analysis for discrete treatment regimens—pharmacologic versus mechanical, pharmacologic versus pharmacologic, pharmacologic and mechanical versus pharmacologic alone—may provide increased clarity with respect to benefit for high-risk patients. Patients, likewise, should be stratified by relative risk for VTE (by spine segment involved, complete vs incomplete injury, time to mobilization), as differing prophylaxis strategies may be appropriate based on risk assessment. Standardization of reporting for complications—particularly bleeding severity—would allow for more objective comparison of relative harms.
Conclusions
There is insufficient evidence to provide discrete recommendations regarding VTE prophylaxis for the specific population of patients presenting with thoracic and lumbar spine injuries. However, the consensus of the work group—on the basis of pooled spinal cord populations—is that thromboprophylaxis is recommended.
Potential Conflicts of Interest
The task force members were required to report all possible conflicts of interest (COIs) prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Review Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chairs reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chairs are given latitude to approve nominations of Task Force members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methods manuscript.
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons and the Section on Disorders of the Spine and Peripheral Nerves in collaboration with the Section on Neurotrauma and Critical Care, which received no funding from outside commercial sources to support the development of this document.
Acknowledgments
The guidelines task force would like to acknowledge the CNS Guidelines Committee for their contributions throughout the development of the guideline and the AANS/CNS Joint Guidelines Review Committee for their review, comments, and suggestions throughout peer review, as well as the contributions of Trish Rehring, MPH, CHES, Senior Manager of Clinical Practice Guidelines for the CNS, and Mary Bodach, MLIS, Guidelines Specialist and Medical Librarian for assistance with the literature searches. Throughout the review process the reviewers and authors were blinded from one another. At this time, the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Maya Babu, MD, MBA, Greg Hawryluk, MD, PhD, Steven Kalkanis, MD, Yi Lu, MD, PhD, Jeffrey J. Olson, MD, Martina Stippler, MD, Cheerag Upadhyaya, MD, MSc, and Robert Whitmore, MD.
REFERENCES
1. Rathore MF, Hanif S, New PW, Butt AW, Aasi MH, Khan SU. The prevalence of deep vein thrombosis in a cohort of patients with spinal cord injury following the Pakistan earthquake of October 2005. Spinal Cord 2008;46:523-526.
2. Powell M, Kirshblum S, O’Connor KC. Duplex ultrasound screening for deep vein thrombosis in spinal cord injured patients at rehabilitation admission. Arch Phys Med Rehabil 1999;80:1044-1046.
3. Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med 1994;331:1601-1606.
4. Lamb GC, Tomski MA, Kaufman J, Maiman DJ. Is chronic spinal cord injury associated with increased risk of venous thromboembolism? J Am Paraplegia Soc 1993;16:153-156.
5. Waring WP, Karunas RS. Acute spinal cord injuries and the incidence of clinically occurring thromboembolic disease. Paraplegia 1991;29:8-16.
6. Watson N. Venous thrombosis and pulmonary embolism in spinal cord injury. Paraplegia 1968;6:113-121.
7. Burns GA, Cohn SM, Frumento RJ, Degutis LC, Hammers L. Prospective ultrasound evaluation of venous thrombosis in high-risk trauma patients. J Trauma 1993;35:405-408.
8. Gündüz S, Ogur E, Mohur H, Somuncu I, Acjksoz E, Ustunsoz B. Deep vein thrombosis in spinal cord injured patients. Paraplegia 1993;31:606-610.
9. Kulkarni JR, Burt AA, Tromans AT, Constable PD. Prophylactic low dose heparin anticoagulant therapy in patients with spinal cord injuries: a retrospective study. Paraplegia 1992;30:169-172.
10. El Masri WS, Silver JR. Prophylactic anticoagulant therapy in patients with spinal cord injury. Paraplegia 1981;19:334-342.
11. Frisbie JH, Sasahara AA. Low dose heparin prophylaxis for deep venous thrombosis in acute spinal cord injury patients: a controlled study. Paraplegia 1981;19:343-346.
12. Myllynen P, Kammonen M, Rokkanen P, Bostman O, Lalla M, Laasonen E. Deep venous thrombosis and pulmonary embolism in patients with acute spinal cord injury: a comparison with nonparalyzed patients immobilized due to spinal fractures. J Trauma 1985;25:541-543.
13. Merli GJ, Crabbe S, Doyle L, Ditunno JF, Herbision GJ. Mechanical plus pharmacological prophylaxis for deep vein thrombosis in acute spinal cord injury. Paraplegia 1992;30:558-562.
14. Rossi EC, Green D, Rosen JS, Spies SM, Jao JS. Sequential changes in factor VIII and platelets preceding deep vein thrombosis in patients with spinal cord injury. Br J Haematol 1980;45:143-151.
15. Winemiller MH, Stolp-Smith KA, Silverstein MD, Therneau TM. Prevention of venous thromboembolism in patients with spinal cord injury: effects of sequential pneumatic compression and heparin. J Spinal Cord Med 1999;22:182-191.
16. Jones T, Ugalde V, Franks P, Zhou H, White RH. Venous thromboembolism after spinal cord injury: incidence, time course, and associated risk factors in 16,240 adults and children. Arch Phys Med Rehabil 2005;86:2240-2247.
17. Worley S, Short C, Pike J, Anderson D, Douglas JA, Thompson K. Dalteparin vs low-dose unfractionated heparin for prophylaxis against clinically evident venous thromboembolism in acute traumatic spinal cord injury: a retrospective cohort study. J Spinal Cord Med 2008;31:379-387.
18. Walsh JJ, Tribe C. Phlebo-thrombosis and pulmonary embolism in paraplegia. Paraplegia 1965;3:209-213.
19. Ploumis A, Ponnappan RK, Bessey JT, Patel R, Vaccaro AR. Thromboprophylaxis in spinal trauma surgery: consensus among spine trauma surgeons. Spine J 2009;9:530-536.
20. Becker DM, Gonzalez M, Gentili A, Eismont F, Green BA. Prevention of deep venous thrombosis in patients with acute spinal cord injuries: use of rotating treatment tables. Neurosurgery 1987;20:675-677.
21. Katz RT, Green D, Sullivan T, Yarkony G. Functional electric stimulation to enhance systemic fibrinolytic activity in spinal cord injury patients. Arch Phys Med Rehabil 1987;68:423-426.
22. Casas ER, Sanchez MP, Arias CR, Masip JP. Prophylaxis of venous thrombosis and pulmonary embolism in patients with acute traumatic spinal cord lesions. Paraplegia 1977;15:209-214.
23. Hachen HJ. Anticoagulant therapy in patients with spinal cord injury. Paraplegia 1974;12:176-187.
24. Green D, Lee MY, Ito VY, et al. Fixed- vs adjusted-dose heparin in the prophylaxis of thromboembolism in spinal cord injury. JAMA 1988;260:1255-1258.
25. Slavik RS, Chan E, Gorman SK, et al. Dalteparin versus enoxaparin for venous thromboembolism prophylaxis in acute spinal cord injury and major orthopedic trauma patients: ‘DETECT’ trial. J Trauma 2007;62:1075-1081; discussion 1081.
26. Chiou-Tan FY, Garza H, Chan KT, et al. Comparison of dalteparin and enoxaparin for deep venous thrombosis prophylaxis in patients with spinal cord injury. Am J Phys Med Rehabil 2003;82:678-685.
27. Thumbikat P, Poonnoose PM, Balasubrahmaniam P, Ravichandran G, McClelland MR. A comparison of heparin/warfarin and enoxaparin thromboprophylaxis in spinal cord injury: the Sheffield experience. Spinal Cord 2002;40:416-420.
28. Green D, Lee MY, Lim AC, et al. Prevention of thromboembolism after spinal cord injury using low-molecular-weight heparin. Ann Intern Med 1990;113:571-574.
29. Spinal Cord Injury Thromboprophylaxis Investigators.Prevention of venous thromboembolism in the acute treatment phase after spinal cord injury: a randomized, multicenter trial comparing low-dose heparin plus intermittent pneumatic compression with enoxaparin. J Trauma 2003;54:1116-1124; discussion 1125-1126.
30. Spinal Cord Injury Thromboprophylaxis Investigators. Prevention of venous thromboembolism in the rehabilitation phase after spinal cord injury: prophylaxis with low-dose heparin or enoxaparin. J Trauma 2003;54:1111-1115.
31. Maxwell RA, Chavarria-Aguilar M, Cockerham WT, et al. Routine prophylactic vena cava filtration is not indicated after acute spinal cord injury. J Trauma 2002;52:902-906.
32. Merli GJ, Herbison GJ, Ditunno JF, et al. Deep vein thrombosis: prophylaxis in acute spinal cord injured patients. Arch Phys Med Rehabil 1988;69:661-664.
33. Ransohoff DF, Pignone M, Sox HC. How to decide whether a clinical practice guideline is trustworthy. JAMA 2013;309:139-140.
34. Green D, Rossi EC, Yao JS, Flinn WR, Spies SM. Deep vein thrombosis in spinal cord injury: effect of prophylaxis with calf compression, aspirin, and dipyridamole. Paraplegia 1982;20:227-234.
35. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 suppl):e227S-e277S.
36. Dhall SS, Hadley MN, Aarabi B, et al. Deep venous thrombosis and thromboembolism in patients with cervical spinal cord injuries. Neurosurgery 2013;72(suppl 2):244-254.
Appendix I. Literature Searches
Search Strategies
PubMed
- Lumbar vertebrae [MeSH] OR Thoracic vertebrae [MeSH]
- Thoracolumbar [TIAB] OR thoraco-lumbar [TIAB] OR thoraco lumbar [TIAB] OR burst [Title]
- Spinal Injuries [MeSH] OR Spinal Cord Injuries [MeSH]
- (Vertebra*[tiab] OR spine[tiab] OR spinal[tiab] OR “spinal cord” [tiab]) AND (Injur*[tiab] OR trauma*[tiab] OR fractur*[tiab] OR dislocation*[tiab])
- #1 OR #2 OR #3 OR #4
- Phlebography[mh] OR D-dimer [title] OR venography [title] OR MR venography [title] OR magnetic resonance venography [title] OR duplex ultrasound [title] OR “Doppler flow measurement” [title] OR Plethysmography, Impedance [MeSH] OR “impedance plethysmography” [title] OR “125I-labeled fibrinogen scanning” [title]
- "Venous Thrombosis"[mh:noexp] OR Thrombophlebitis[mh] OR "Venous Thromboembolism"[mh] OR dvt [title] OR vte [title] OR thrombos* [title] OR thrombophleb* [title] OR thromboembol* [title] OR thromboprophyla* [title] OR "Pulmonary embolism"[mh] OR ((pulmonary [title] OR lung [title] OR lungs [title]) AND (infarct* [title] OR embol* [title] OR clot [title] OR clots [title] OR bloodclot* [title]))
- Thrombolytic therapy [mh] OR chemoprophyla*[tiab] OR Anticoagulants[mh] OR anticoagulants[pa] OR anticoagul*[title] OR "fibrinolytic agents"[mh] OR "fibrinolytic agents"[pa] OR antithrombo*[title] OR “thrombolytic therapy”[mh] OR thrombolytic*[title] OR antiplatelet*[title] OR anti-platelet*[title] OR "platelet aggregation inhibitors"[mh] OR heparin[mh] OR heparin*[title] OR enoxaparin[title] OR lovenox[title] OR plavix[title] OR coumadin[title] OR clopidogrel[nm] OR warfarin[mh] OR warfarin*[title] OR fragmin[title] OR dalteparin[title] OR innohep[title] OR tinzaparin[nm] OR arixtra[title] OR fondaparinux[nm] OR "factor Xa inhibitor"[title] OR angiomax[title] OR bivalirudin[nm] OR refludan[title] OR aspirin[mh] OR aspirin[title] OR lepirudin[nm] OR iprivask[title] OR desirudin[nm] OR pradaxa[title] OR dabigatran[title] OR "dabigatran etexilate"[nm] OR xarelto[title] OR rivaroxaban[nm] OR YM150[tw] OR LY517717[tw] OR apixaban[tw]
- "Vena cava filters"[mh] OR ("Vena cava, inferior"[mh] AND "Filtration/instrumentation"[mh] AND 1972:1990[mhda]) OR "vena cava"[title] OR ivc [title]
- "stockings, compression"[mh] OR (compression[title] AND (sequential[title] OR stocking*[title] OR device*[title] OR (bandages[mh] AND 1970:2006[mhda]))) OR "Intermittent Pneumatic Compression Devices"[mh] OR (foot[title] AND (pump[title] OR pumps[title])) OR ((pneumatic[tiab] OR leg[title] OR calf[title]) AND compression[title]) OR (mechanical[title] AND prophyla*[title]) OR Ted hose [title]
- #6 OR #7 OR #8 OR #9 OR #10
- #5 AND #11
- (animal [MeSH] NOT human [MeSH]) OR cadaver [MeSH] OR cadaver* [Titl] OR comment [PT] OR letter [PT] OR editorial [PT] OR addresses [PT] OR news [PT] OR “newspaper article” [PT] OR case reports [PT] OR case report [Title]
- #12 NOT #3
- osteoporosis [MH] OR osteoporotic fractures [MH] OR osteoporo* [TITLE] OR spinal neoplasms [MH] OR tumor* [TITLE] OR tumour* [TITLE] OR malignan* [TITLE]
- #14 NOT #15
- #16 AND English [Lang]
- #17 AND ("1946/01/01"[PDAT] : "2015/03/31"[PDAT])
Cochrane Library
- Lumbar vertebrae: MeSH descriptor, explode all trees
- Thoracic vertebrae: MeSH descriptor, explode all trees
- Spinal Injuries: MeSH descriptor
- Spinal Cord Injuries: MeSH descriptor
- (Thoracolumbar OR thoraco-lumbar OR thoraco lumbar OR burst) NEAR/4 (Injur* OR trauma* OR fractur* OR dislocation*):ti,ab,kw
- 1 OR 2 OR 3 OR 4 OR 5
- Spinal Injury OR Thoracolumbar Root search
- Phlebography: MeSH descriptor, explode all trees
- Plethysmography, Impedance: MeSH descriptor, explode all trees
- D-dimer or venography or MR venography or duplex ultrasound or “Doppler flow measurement” or “impedance plethysmography” or “125I-labeled fibrinogen scanning”:ti
- "Venous Thrombosis": MeSH descriptor, this term only
- Thrombophlebitis: MeSH descriptor, explode all trees
- "Venous Thromboembolism": MeSH descriptor, explode all trees
- "Pulmonary embolism": MeSH descriptor, explode all trees
- dvt or vte or thrombos* or thrombophleb* or thromboembol* or thromboprophyla*:ti
- Thrombolytic therapy: MeSH descriptor, explode all trees
- Anticoagulants : MeSH descriptor, explode all trees
- "fibrinolytic agents”: MeSH descriptor, explode all trees
- "platelet aggregation inhibitors": MeSH descriptor, explode all trees
- Heparin: MeSH descriptor, explode all trees
- Warfarin: MeSH descriptor, explode all trees
- Aspirin: MeSH descriptor, explode all trees
- chemoprophyla*or anticoagul* or antithrombo* or thrombolytic*or antiplatelet*or anti-platelet*or heparin* or enoxaparin or lovenox or Plavix or Coumadin or clopidogrel or warfarin* or fragmin or dalteparin or innohep or arixtra or fondaparinux or "factor Xa inhibitor" or angiomax or bivalirudin or refludan or aspirin or lepirudin or iprivask or desirudin or pradaxa or dabigatran or "dabigatran etexilate" or xarelto or rivaroxaban or YM150 or LY517717 or apixaban:ti
- "Vena cava filters": MeSH descriptor, explode all trees
- "vena cava filter” or ivc:ti
- "stockings, compression": MeSH descriptor, explode all trees
- "Intermittent Pneumatic Compression Devices": MeSH descriptor, explode all trees
- compression stocking* or compression device* or foot pump or foot pumps or mechanical prophyla* or Ted hose:ti
- #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22
- #1 and #23—102
- osteoporo* or tumor* or malignan*:ti
- osteoporosis : MeSH descriptor, explode all trees
- osteoporotic fractures: MeSH descriptor, explode all trees
- spinal neoplasms: MeSH descriptor, explode all trees
- #25 or #26 or #27 or #28
- #24 not #29
Appendix II. Article Inclusions and Exclusions
Included and Excluded Articles Flowchart
Appendix III. Rating Evidence Quality
Levels of Evidence for Primary Research Questiona
|
Types of studies
|
|
|
Therapeutic studies – Investigating the results of treatment
|
Prognostic studies – Investigating the effect of a patient characteristic on the outcome of disease
|
Diagnostic studies – Investigating a diagnostic test
|
Economic and decision analyses – Developing an economic or decision model
|
|
Level I
|
- High-quality randomized trial with statistically significant difference or no statistically significant difference but narrow confidenceintervals
- Systematic reviewb of level I RCTs (and study results were homogenousc)
|
- High-quality prospective studyd (all patients were enrolled at the same point in their disease with
≥80% follow-up of enrolled patients)
- Systematic reviewb of level I studies
|
- Testing of previously developed diagnostic criteria on consecutive patients (with universally applied reference “gold” standard)
- Systematic reviewb of level I studies
|
- Sensible costs and alternatives; values obtained from many studies; with multiway sensitivity analyses
- Systematic reviewb of level I studies
|
|
Level II
|
- Lesser quality RCT (e.g., ≤80% follow-up, no blinding, or improper randomization)
- Prospectived comparative studye
- Systematic reviewb of level II studies or level I studies with inconsistent results
|
- Retrospectivef study
- Untreated controls from an RCT
- Lesser quality prospective study (e.g., patients enrolled at different points in their disease or
≤80% follow-up)
- Systematic reviewb of level II studies
|
- Development of diagnostic criteria on consecutive patients (with universally applied reference “gold” standard)
- Systematic reviewb of level II studies
|
- Sensible costs and alternatives; values obtained from limited studies; with multiway sensitivity analyses
- Systematic reviewb of level II studies
|
|
Level III
|
- Case control studyg
- Retrospectivef comparative studye
- Systematic reviewb of level III studies
|
|
- Study of non consecutive patients; without consistently applied reference “gold” standard
- Systematic reviewb of level III studies
|
- Analyses based on limited alternatives and costs; and poor estimates
- Systematic reviewb of level III studies
|
|
Level IV
|
Case seriesh
|
Case series
|
- Case-control study
- Poor reference standard
|
- Analyses with no sensitivity analyses
|
RCT, Randomized controlled trial.
aA complete assessment of quality of individual studies requires critical appraisal of all aspects of the study design.
bA combination of results from ≥2 previous studies.
cStudies provided consistent results.
dStudy was started before the first patient enrolled.
ePatients treated one way (e.g., instrumented arthrodesis) compared with a group of patients treated in another way (e.g., unsintrumented arthrodesis) at the same institution.
fThe study was started after the first patient enrolled.
gPatients identified for the study based on their outcome, called “cases” (e.g., pseudoarthrosis) are compared to those who did not have outcome, called “controls” (e.g., successful fusion).
hPatients treated one way with no comparison group of patients treated in another way.
Appendix IV. Linking Levels of Evidence to Grades of Recommendation
|
Grade of Recommendation
|
Standard Language
|
Levels of Evidence
|
|
A
|
Recommended
|
Two or more consistent level I studies
|
|
B
|
Suggested
|
One level I study with additional supporting level II or III studies
|
Two or more consistent level II or III studies
|
|
C
|
Is an option
|
One level I, II, or III study with supporting level IV studies
|
Two or more consistent level IV studies
|
|
Insufficient
(insufficient or conflicting evidence)
|
Insufficient evidence to make recommendation for or against
|
A single level I, II, III, or IV study without other supporting evidence
|
>1 study with inconsistent findingsa
|
aNote that in the presence of multiple consistent studies, and a single outlying, inconsistent study, the Grade of Recommendation will be based on the level of the consistent studies.
Appendix V. Criteria Grading the Evidence
The task force used the criteria provided below to identify the strengths and weaknesses of the studies included in this guideline. Studies containing deficiencies were downgraded one level (no further downgrading allowed, unless so severe that study had to be excluded). Studies with no deficiencies based on study design and contained clinical information that dramatically altered current medical perceptions of topic were upgraded.
- Baseline study design (i.e., therapeutic, diagnostic, prognostic) determined to assign initial level of evidence.
- Therapeutic studies reviewed for following deficiencies:
- Failure to provide a power calculation for an RCT;
- High degree of variance or heterogeneity in patient populations with respect to presenting diagnosis/demographics or treatments applied;
- <80% of patient follow-up;
- Failure to utilize validated outcomes instrument;
- No statistical analysis of results;
- Cross over rate between treatment groups of >20%;
- Inadequate reporting of baseline demographic data;
- Small patient cohorts (relative to observed effects);
- Failure to describe method of randomization;
- Failure to provide flowchart following patients through course of study (RCT);
- Failure to account for patients lost to follow-up;
- Lack of independent post-treatment assessment (e.g., clinical, fusion status, etc.);
- Utilization of inferior control group:
- Historical controls;
- Simultaneous application of intervention and control within same patient.
- Failure to standardize surgical/intervention technique;
- Inadequate radiographic technique to determine fusion status (e.g., static radiographs for instrumented fusion).
- Methodology of diagnostic studies reviewed for following deficiencies:
- Failure to determine specificity and sensitivity;
- Failure to determine inter- and intraobserver reliability;
- Failure to provide correlation coefficient in the form of kappa values.
- Methodology of prognostic studies reviewed for following deficiencies:
- High degree of variance or heterogeneity in patient populations with respect to presenting diagnosis/demographics or treatments applied;
- Failure to appropriately define and assess independent and dependent variables (e.g., failure to use validated outcome measures when available).
Appendix VI. Evidence Table
|
Author, Year
|
Level of Evidence
|
Task Force Conclusions Relative to Question and Rationale for Evidence Grading
|
|
Green et al, 1982
|
II
|
This paper provides evidence that while external pneumatic calf compression decreases incidence of deep venous thrombosis, the combination of mechanical and chemical prophylaxis (aspirin plus dipyridamole) provides greater reduction
|
© Congress of Neurological Surgeons
Source: Neurosurgery, September 6, 2018