Guidelines on the Evaluation and Treatment of Patients with Thoracolumbar Spine Trauma
5. Pharmacological Treatment
download pdf Neurosurgery, 2018
Sponsored by: Congress of Neurological Surgeons and the Section on Disorders of the Spine and Peripheral Nerves in collaboration with the Section on Neurotrauma and Critical Care
Endorsed by: The Congress of Neurological Surgeons (CNS) and the American Association of Neurological Surgeons (AANS)
Paul M. Arnold, MD,1 Paul A. Anderson, MD,2 John H. Chi, MD, MPH,3 Andrew T. Dailey, MD,4 Sanjay S. Dhall, MD,5 Kurt M. Eichholz, MD,6 James S. Harrop, MD,7 Daniel J. Hoh, MD,8 Sheeraz Qureshi, MD, MBA,9 Craig H. Rabb, MD,10 P. B. Raksin, MD,11 Michael G. Kaiser, MD,12 and John E. O'Toole, MD, MS13
1. Department of Neurosurgery, University of Kansas School of Medicine, Kansas City, Kansas
2. Department of Orthopedics and Rehabilitation, University of Wisconsin, Madison, Wisconsin
3. Department of Neurosurgery, Harvard Medical School, Brigham and Women’s Hospital, Boston, Massachusetts
4. Department of Neurosurgery, University of Utah, Salt Lake City, Utah
5. Department of Neurological Surgery, University of California, San Francisco, San Francisco, California
6. St. Louis Minimally Invasive Spine Center, St. Louis, Missouri
7. Departments of Neurological Surgery and Orthopedic Surgery, Thomas Jefferson University, Philadelphia, Pennsylvania
8. Lillian S. Wells Department of Neurological Surgery, University of Florida, Gainesville, Florida
9. Department of Orthopaedic Surgery, Weill Cornell Medical College, New York, New York
10. Department of Neurosurgery, University of Utah, Salt Lake City, Utah
11. Division of Neurosurgery, John H. Stroger, Jr. Hospital of Cook County and Department of Neurological Surgery, Rush University Medical Center, Chicago, Illinois
12. Department of Neurosurgery, Columbia University, New York, New York
13. Department of Neurological Surgery, Rush University Medical Center, Chicago, Illinois
Correspondence:
Paul M. Arnold, M.D
3901 Rainbow Blvd. MS 3021 Kansas City, KS 66160
Professor of Neurosurgery and Vice-Chair for Research
University of Kansas School of Medicine
Email: parnold@kumc.edu
Keywords: Thoracic spinal cord injury; lumbar spinal cord injury; thoracolumbar spine trauma
Abbreviations
AANS – American Association of Neurological Surgeons
CNS – Congress of Neurological Surgeons
MPSS – Methylprednisolone sodium succinate
NASCIS – National Acute Spinal Cord Injury Study
SCI – Spinal cord injury
No part of this article has been published or submitted for publication elsewhere.
ABSTRACT
Background: In the United States, there are approximately 17,000 new spinal cord injuries (SCIs) annually, with most occurring in patients <45 years of age. Annual direct costs in the United States are estimated at $14 billion to $18 billion, which do not include the devastating psychological consequences and lost productivity associated with these injuries. In the past 25 years, there has been intense scientific interest in finding an effective treatment for SCIs; however, pharmacologic therapy has not kept pace with the medical treatment of thoracolumbar SCIs.
Objective: We sought to conduct a systematic review of the literature and establish evidence-based guidelines on the pharmacologic management of acute traumatic thoracolumbar SCI.
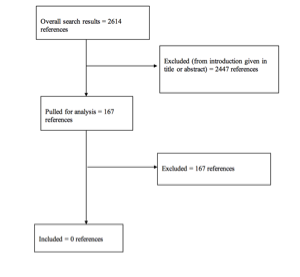
Methods: A systematic literature search using the PubMed database was conducted for articles published between January 1, 1946 and March 31, 2015, yielding 2614 articles. One hundred sixty-seven articles were selected for full-text review and analysis. A level of medical evidence was assigned to each article, and the strength of recommendation assessed according to a modified version of the North American Spine Society’s guideline development methodology.
Results: No study clearly showed that a particular pharmacologic agent was advantageous in the treatment of thoracolumbar SCI. No study reached the inclusion threshold of 80% thoracolumbar spinal cord injury patients, and most studies had >50% cervical SCI. Therefore, all studies were excluded.
Conclusion: Based on the data found in the literature, there are no agents that are specifically recommended for the pharmacologic treatment of acute thoracolumbar SCI.
RECOMMENDATIONS
|
Question
|
|
Does the administration of a specific pharmacologic agent (e.g., methylprednisolone) improve clinical outcomes in patients with thoracic and lumbar fractures and spinal cord injury?
|
|
Recommendation
|
|
There is insufficient evidence to make a recommendation; however, the task force concluded, in light of previously published data and guidelines, that the complication profile should be carefully considered when deciding on the administration of methylprednisolone.
|
|
Strength of Recommendation: Grade: Insufficient
|
INTRODUCTION
Goals and Rationale
This clinical guideline has been created to improve patient care by outlining the appropriate information gathering and decision-making processes involved in the evaluation and treatment of patients with thoracolumbar spinal cord injuries. The surgical management of these patients often takes place under a variety of circumstances and by various clinicians. This guideline has been created as an educational tool to guide qualified physicians through a series of diagnostic and treatment decisions to improve the quality and efficiency of care.
Spinal cord injuries (SCIs) remain a devastating clinical problem, both to the individual patient and to society as a whole. Males and individuals <45 years of age are disproportionately affected, and depending on the level and severity of injury, the lifetime cost of care for a single patient may reach $4 million USD.1 The most common cause of SCI is motor vehicle accidents, followed by falls, acts of violence (primarily gunshot wounds), and sports/recreational activities.1 The annual incidence of SCI in the United States is approximately 54 cases per million people, or approximately 17,000 new SCI cases each year, not including those who die at the scene of the accident.1 The prevalence in the United States in 2016 is estimated to be approximately 282,000 people, with a range from 243,000 to 347,000 persons.1
There are currently few options available for the treatment of SCI. Surgical management includes decompression of the injured spinal cord and fixation and fusion of the spine with prevention of secondary injury, but surgery does not directly address the initial insult. Improvements in the medical management of spinal cord injury patients now provide the opportunity for a near-normal life span.
An increased understanding of the pathophysiology of SCI has led to the initiation of several recent pharmacologic clinical trials, including National Acute Spinal Cord Injury Study (NASCIS) I and II, the Sygen (GM-1 ganglioside) trials, riluzole, minocycline, and others. However, to date, none of these drugs have been shown to significantly improve neurologic outcome following acute SCI, and the use of methylprednisolone for SCI remains controversial.
The purpose of this guideline was to review the literature regarding the efficacy of pharmacologic agents in the management of acute thoracolumbar SCI and to determine if the administration of a specific pharmacologic agent (e.g., methylprednisolone) improved clinical outcomes in patients with thoracic and lumbar fractures, and spinal cord injury.
Methods
The guidelines task force initiated a systematic review of the literature relevant to the diagnosis and treatment of patients with thoracolumbar SCIs. Through objective evaluation of the evidence and transparency in the process of making recommendations, this evidence-based clinical practice guideline was developed for the diagnosis and treatment of adult patients with thoracolumbar injury. These guidelines are developed for educational purposes to assist practitioners in their clinical decision-making processes. Additional information about the methods used in this systematic review can be found in the introduction and methodology chapter.
Literature Search
The task force members identified search terms/parameter and a medical librarian implemented the literature search, consistent with the literature search protocol (see Appendix I), using the National Library of Medicine PubMed database and the Cochrane Library (which included the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effect, the Cochrane Central Register of Controlled Trials, the Health Technology Assessment Database, and the National Health Service Economic Evaluation Database) for the period from January 1, 1946 to March 31, 2015, using the search strategies provided in Appendix I.
RESULTS
The literature search yielded 2614 abstracts. Task force members reviewed all abstracts yielded from the literature search and identified the literature for full text review and extraction, addressing the clinical questions, in accordance with the literature search protocol (Appendix I). Task force members identified the best research evidence available to answer the targeted clinical questions. When level I, II, or III literature was available to answer specific questions, the task force did not review level IV studies.
The task force selected 167 articles for full-text review. Of these, all studies were rejected for not meeting inclusion criteria or for being off topic. No studies were selected for systematic review (Appendix II).
Inclusion/Exclusion Criteria
Articles were retrieved and included only if they met specific inclusion/exclusion criteria. These criteria were also applied to articles provided by guideline task force members who supplemented the electronic database searches with articles from their own files. To reduce bias, these criteria were specified before conducting the literature searches.
Articles that do not meet the following criteria were, for the purposes of this evidence-based clinical practice guideline, excluded. To be included as evidence in the guideline, an article had to be a report of a study that:
- Investigated patients with thoracolumbar injuries;
- Included patients ≥18 years of age;
- Enrolled ≥80% of thoracolumbar injuries (studies with mixed patient populations were included if they reported results separately for each group/patient population);
- Was a full article report of a clinical study;
- Was not an internal medical records review, meeting abstract, historical article, editorial, letter, or commentary;
- Appeared in a peer-reviewed publication or a registry report;
- Enrolled ≥10 patients per arm per intervention (20 total) for each outcome;
- Included only human subjects;
- Was published in or after 1946 through March 31, 2015;
- Quantitatively presented results;
- Was not an in vitro study;
- Was not a biomechanical study;
- Was not performed on cadavers;
- Was published in English;
- Was not a systematic review, meta-analysis, or guideline developed by others[1];
- Was a case series (therapeutic study) where higher level evidence exists.
Rating Quality of Evidence
The guideline task force used a modified version of the North American Spine Society’s evidence-based guideline development methodology. The North American Spine Society methodology uses standardized levels of evidence (Appendix III) and grades of recommendation (Appendix IV) to assist practitioners in easily understanding the strength of the evidence and recommendations within the guidelines. The levels of evidence range from level I (high quality randomized controlled trial) to level IV (case series). Grades of recommendation indicate the strength of the recommendations made in the guideline based on the quality of the literature. Levels of evidence have specific criteria and are assigned to studies before developing recommendations. Recommendations are then graded based upon the level of evidence. To better understand how levels of evidence inform the grades of recommendation and the standard nomenclature used within the recommendations, see Appendix IV.
Guideline recommendations were written using a standard language that indicates the strength of the recommendation. “A” recommendations indicate a test or intervention is 2 “recommended”; “B” recommendations “suggest” a test or intervention; “C” recommendations indicate a test or intervention or “is an option.” “Insufficient evidence” statements clearly indicate that “there is insufficient evidence to make a recommendation for or against” a test or intervention. Task force consensus statements clearly state that “in the absence of reliable evidence, it is the task force’s opinion that” a test or intervention may be considered. Both the levels of evidence assigned to each study and the grades of each recommendation were arrived at by consensus of the workgroup employing up to three rounds of voting when necessary.
In evaluating studies as to levels of evidence for this guideline, the study design was interpreted as establishing only a potential level of evidence. For example, a therapeutic study designed as a randomized controlled trial would be considered a potential level I study. The study would then be further analyzed as to how well the study design was implemented and significant shortcomings in the execution of the study would be used to downgrade the levels of evidence for the study’s conclusions (see Appendix V for additional information and criteria).
Revision Plans
In accordance with the Institute of Medicine’s standards for developing clinical practice guidelines and criteria specified by the National Guideline Clearinghouse, the task force will monitor related publications after the release of this document and will revise the entire document or specific sections “if new evidence shows that a recommended intervention causes previously unknown substantial harm; that a new intervention is significantly superior to a previously recommended intervention from an efficacy or harms perspective; or that a recommendation can be applied to new populations.”2 In addition, the task force will confirm within 5 years from the date of publication that the content reflects current clinical practice and the available technologies for the evaluation and treatment for patients with thoracolumbar spinal cord injuries.
DISCUSSION
|
Question
|
|
Does the administration of a specific pharmacologic agent (e.g., methylprednisolone) improve clinical outcomes in patients with thoracic and lumbar fractures and spinal cord injury?
|
|
Recommendation
|
|
There is insufficient evidence to make a recommendation; however, the task force concluded, in light of previously published guidelines and data, that the complication profile should be carefully considered when deciding on the administration of methylprednisolone.
|
|
Level of Evidence: Insufficient
|
Methylprednisolone Sodium Succinate
Methylprednisolone sodium succinate (MPSS) is by far the most extensively studied pharmacologic agent used to treat patients with acute SCI. There have been 3 NASCIS studies performed between 1980 and 1998. The first study (NASCIS I) compared low-dose MPSS versus high-dose MPSS, and the short- and long-term results showed no significant neurologic difference between the 2 groups. There were significantly more complications in the high-dose group, including a 3-times higher rate of wound infection.3
The second NASCIS study (NASCIS II) compared a higher dose MPSS with naloxone and a placebo control.4 Patients in this study who received MPSS were loaded with 30 mg/kg and then received 5.4 mg/kg/hour for the next 23 hours. In analyzing all patients who were randomized within 24 hours of injury, there was no improvement in neurologic outcome in patients receiving MPSS. However, patients who received the drug within 8 hours of injury (a post hoc analysis) had improved motor and sensory scores at 6 months after injury, especially those with more severe injury. However, the sensory gains were lost at the 1-year endpoint. Again, there was also a trend toward higher rates of complications in the MPSS group.4
The last NASCIS study (NASCIS III) compared a 24-hour continuous infusion of MPSS versus a 48-hour infusion. A third arm of the study looked at tirilizad mesylate. This trial showed no long-lasting neurologic benefit of MPSS. Post hoc review noted that patients receiving MPSS bolus 3 to 8 hours after injury had short-term neurologic improvement when given MPSS for 48 hours, but these gains were lost at 1 year. Patients undergoing 48-hour MPSS infusion also had substantially higher infectious complications and a higher death rate than the 24-hour group.5 Xu et al6 prospectively looked at acute SCI outcomes in patients receiving MPSS and GM-I, and compared them to two control groups, either MPSS or GM-I alone. This study showed that the combined drug group did better than either of the controls, but this was a small study with no validated outcome and poor data presentation.
One other prospective study looked at the effects of MPSS for acute SCI. Pointillart et al7 showed no benefit of MPSS. This study had a small number of patients and methodological flaws, and thus the value of these data are limited.
The use of MPSS as an adjunct to the management of acute SCI remains controversial because of the paucity of functional neurologic benefits, the high rate of complications in the MPSS group, and the use of post hoc analysis to determine benefits in a subsection of the patient population. The AANS/CNS Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injuries were specific, noting that the use of MPSS was not recommended for the treatment of acute SCI.8 The authors went on to note that the U.S. Food and Drug Administration does not approve its use for SCI, and that there was no class I or class II medical evidence supporting MPSS for this diagnosis. They also noted the higher rate of complications, including death, associated with MPSS.
GM-1 Ganglioside (Sygen)
There have been 2 prospective randomized clinical trials that investigated the efficacy of Sygen, a GM-1 ganglioside, in the treatment of acute SCI. The first study was performed at a single institution with 37 patients.9 All patients received MPSS before randomization, and GM-1 patients received the drug 72 hours after injury. Data showed that the GM-1 patients had significant neurologic recovery compared to the MPSS-only patients, which was the impetus for a larger multicenter trial. However, this larger trial failed to show a significant difference in neurologic outcome compared to the MPSS patients, despite a trend for earlier recovery in the GM-1 patients.10 The study was also criticized for not having a true control group.
The calcium-channel blocker nimodipine was evaluated in an acute SCI trial along with a placebo and MPSS. A fourth arm of this study included MPSS and nimodipine. There were only 100 patients in the study, and no treatment group showed any improved neurologic function compared to the placebo group.7
Several other agents, including minocycline,11 ProCord,12,13 BA-210/Cethrin,14 recombinant human erythropoietin,15,16 riluzole,17 and granulocyte colony-stimulating factor18 have also been investigated for use following traumatic SCI. These trials have been pilot studies, nonrandomized, or too underpowered to show any current benefit.
Future Research
Despite intense interest and the completion of several well-designed prospective randomized clinical trials, no pharmacologic agent has been shown to improve neurologic outcomes in acute SCI. However, there are several current clinical trials investigating both pharmacologic and nonpharmacologic agents, and there is optimism that 1 of these therapies will be efficacious, offering hope for the treatment of this devastating injury. One of the current studies underway include the In Vivo product, a scaffold that is placed intradurally in patients with ASIA thoracic spinal cord injury. Early results19 from this trial were encouraging, and data on the first 8 patients were presented at the CNS 2016 meeting in San Diego.
CONCLUSIONS
For the past 30 years, intense research has been focused on identifying an effective pharmacologic or cell-based treatment for patients with SCIs. There have been significant advances at the molecular and preclinical levels in our understanding regarding the pathophysiology of SCI, but these advances have not translated to an effective treatment paradigm that will improve neurologic outcome. Although several new potential therapies are currently under investigation, an effective treatment for acute SCI remains elusive.
Potential Conflicts of Interest
The task force members were required to report all possible conflicts of interest (COIs) prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Review Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chairs reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chairs are given latitude to approve nominations of Task Force members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methods manuscript.
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons and the Section on Disorders of the Spine and Peripheral Nerves in collaboration with the Section on Neurotrauma and Critical Care, which received no funding from outside commercial sources to support the development of this document.
Acknowledgments
The guidelines task force would like to acknowledge the CNS Guidelines Committee for their contributions throughout the development of the guideline and the AANS/CNS Joint Guidelines Review Committee for their review, comments, and suggestions throughout peer review, as well as the contributions of Trish Rehring, MPH, CHES, Senior Manager of Clinical Practice Guidelines for the CNS, and Mary Bodach, MLIS, Guidelines Specialist and Medical Librarian for assistance with the literature searches. Throughout the review process the reviewers and authors were blinded from one another. At this time, the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Maya Babu, MD, MBA, Greg Hawryluk, MD, PhD, Steven Kalkanis, MD, Yi Lu, MD, PhD, Jeffrey J. Olson, MD, Martina Stippler, MD, Cheerag Upadhyaya, MD, MSc, and Robert Whitmore, MD.
REFERENCES
1. National Spinal Cord Injury Statistical Center website. Facts and figures at a glance. Available at: https://www.nscisc.uab.edu/Public/Facts%202016.pdf. Accessed January 17, 2017.
2. Ransohoff DF, Pignone M, Sox HC. How to decide whether a clinical practice guideline is trustworthy. JAMA 2013;309:139-140.
3. Bracken MB, Collins WF, Freeman DF, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA 1984;251:45-52.
4. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med 1990;322:1405-1411.
5. Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 1997;277:1597-1604.
6. Xu D, Yang L, Li Y, Sun Y. Clinical study of ganglioside (GM) combined with methylprednisolone (MP) for early acute spinal injury. Pak J Pharm Sci 2015;28(2 suppl):701-704.
7. Pointillart V, Petitjean ME, Wiart L, et al. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord 2000;38:71-76.
8. Hurlbert RJ, Hadley MN, Walters BC, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery 2013;72(suppl 2):93-105.
9. Geisler FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal-cord injury--a randomized, placebo-controlled trial with GM-1 ganglioside. N Engl J Med 1991;324:1829-1838.
10. Geisler FH, Coleman WP, Grieco G, Poonian D. The Sygen multicenter acute spinal cord injury study. Spine 2001;26(24 suppl):S87-S98.
11. Casha S, Zygun D, McGowan MD, Bains I, Yong VW, Hurlbert RJ. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 2012;135(pt 4):1224-1236.
12. Assina R, Sankar T, Theodore N, et al. Activated autologous macrophage implantation in a large-animal model of spinal cord injury. Neurosurg Focus 2008;25:E3.
13. Knoller N, Auerbach G, Fulga V, et al. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J Neurosurg Spine 2005;3:173-181.
14. Fehlings MG, Theodore N, Harrop J, et al. A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotrauma 2011;28:787-796.
15. Alibai E, Zand F, Rahimi A, Rezaianzadeh A. Erythropoietin plus methylprednisolone or methylprednisolone in the treatment of acute spinal cord injury: a preliminary report. Acta Med Iran 2014;52:275-279.
16. Costa DD, Beghi E, Carignano P, et al. Tolerability and efficacy of erythropoietin (EPO) treatment in traumatic spinal cord injury: a preliminary randomized comparative trial vs. methylprednisolone (MP). Neurol Sci 2015;36:1567-1574.
17. Grossman RG, Fehlings MG, Frankowski RF, et al. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma 2014;31:239-255.
18. Kamiya K, Koda M, Furuya T, et al. Neuroprotective therapy with granulocyte colony-stimulating factor in acute spinal cord injury: a comparison with high-dose methylprednisolone as a historical control. Eur Spine J 2015;24:963-967.
19. Layer RT, Ulich TR, Coric D, et al. New clinical-pathological classification of intraspinal injury following traumatic acute complete thoracic spinal cord injury: Postdurotomy/myelotomy observations from the INSPIRE trial. Neurosurgery 2017;64(CN_suppl_1):105-109.
Appendix I. Literature Searches
Search Strategies
PubMed
- Lumbar vertebrae [MeSH] OR Thoracic vertebrae [MeSH]
- Thoracolumbar [TIAB] OR thoraco-lumbar [TIAB] OR thoraco lumbar [TIAB] OR burst [Title]
- Spinal Injuries [MeSH] OR Spinal Cord Injuries [MeSH]
- (Vertebra*[tiab] OR spine[tiab] OR spinal[tiab] OR “spinal cord” [tiab]) AND (Injur*[tiab] OR trauma*[tiab] OR fractur*[tiab] OR dislocation*[tiab])
- #1 OR #2 OR #3 OR #4
- Steroids [Mesh] OR Neuroprotective agents [Mesh] OR neuroprotective agents [PA] OR anti-inflammatory agents [Mesh] OR anti-inflammatory agents [PA] OR glucocorticoids [Mesh] OR glucocorticoids [PA] OR gangliosides [Mesh]
- Steroid* OR Glucocorticoid* OR betamethasone* OR cortisone* OR hydrocortisone* OR triamcinolone* OR corticosteroid* OR Methylprednisolone* OR Urbason OR Medrol OR Solu-Medrol OR Dexamethasone* OR prednisone* OR prednisolone* OR hydrocortisone* OR cortisone acetate OR ganglioside* OR GM1 OR GM-1 OR GM 1 [TITLE]
- #6 OR #7
- #5 AND #8
- (animals [MeSH] NOT humans [MeSH]) OR cadaver [MeSH] OR cadaver* [Titl] OR comment [PT] OR letter [PT] OR editorial [PT] OR addresses [PT] OR news [PT] OR “newspaper article” [PT] OR Case Reports [PT]
- #9 NOT #10
- osteoporosis [MH] OR osteoporotic fractures [MH] OR osteoporo* [TITLE] OR spinal neoplasms [MH] OR tumor* [TITLE] OR tumour* OR malignan* [TITLE] OR Cervical [TITLE]
- #11 NOT #12
- #13 AND English [Lang]
- #14 AND ("1946/01/01"[PDAT] : "2015/03/31"[PDAT])
Cochrane Library
1. Lumbar vertebrae: MeSH descriptor, explode all trees
2.Thoracic vertebrae: MeSH descriptor, explode all trees
3. #1 OR #2
4. Spinal Injuries: MeSH descriptor
5. Spinal Cord Injuries: MeSH descriptor
6. #4 OR #5
7. #3 AND #6
8.(Thoracolumbar OR thoraco-lumbar OR thoraco lumbar OR burst) NEAR/4 (Injur* OR trauma* OR fractur* OR dislocation*):ti,ab,kw
9. Lumbar vertebrae/injuries: MeSH descriptor, explode all trees
10. Thoracic vertebrae/injuries: MeSH descriptor, explode all trees
11. #9 OR #10
12. #7 OR #8 OR #11
13.mh osteoporosis or mh osteoporotic fractures or mh spinal neoplasms
14. osteoporo* or tumor* or malignan*:ti
15. #13 OR #14
16. #12 NOT #15
Appendix II. Article Inclusions and Exclusions
Included and Excluded Articles Flowchart
Appendix III. Rating Evidence Quality
Levels of Evidence for Primary Research Questiona
|
Types of studies
|
|
|
Therapeutic studies – Investigating the results of treatment
|
Prognostic studies – Investigating the effect of a patient characteristic on the outcome of disease
|
Diagnostic studies – Investigating a diagnostic test
|
Economic and decision analyses – Developing an economic or decision model
|
|
Level I
|
- High-quality randomized trial with statistically significant difference or no statistically significant difference but narrow confidenceintervals
- Systematic reviewb of level I RCTs (and study results were homogenousc)
|
- High-quality prospective studyd (all patients were enrolled at the same point in their disease with
≥80% follow-up of enrolled patients)
- Systematic reviewb of level I studies
|
- Testing of previously developed diagnostic criteria on consecutive patients (with universally applied reference “gold” standard)
- Systematic reviewb of level I studies
|
- Sensible costs and alternatives; values obtained from many studies; with multiway sensitivity analyses
- Systematic reviewb of level I studies
|
|
Level II
|
- Lesser quality RCT (e.g., ≤80% follow-up, no blinding, or improper randomization)
- Prospectived comparative studye
- Systematic reviewb of level II studies or level I studies with inconsistent results
|
- Retrospectivef study
- Untreated controls from an RCT
- Lesser quality prospective study (e.g., patients enrolled at different points in their disease or
≤80% follow-up)
- Systematic reviewb of level II studies
|
- Development of diagnostic criteria on consecutive patients (with universally applied reference “gold” standard)
- Systematic reviewb of level II studies
|
- Sensible costs and alternatives; values obtained from limited studies; with multiway sensitivity analyses
- Systematic reviewb of level II studies
|
|
Level III
|
- Case control studyg
- Retrospectivef comparative studye
- Systematic reviewb of level III studies
|
|
- Study of non consecutive patients; without consistently applied reference “gold” standard
- Systematic reviewb of level III studies
|
- Analyses based on limited alternatives and costs; and poor estimates
- Systematic reviewb of level III studies
|
|
Level IV
|
Case seriesh
|
Case series
|
- Case-control study
- Poor reference standard
|
- Analyses with no sensitivity analyses
|
RCT, Randomized controlled trial.
study design.
bA combination of results from ≥2 previous studies.
cStudies provided consistent results.
dStudy was started before the first patient enrolled.
ePatients treated one way (e.g., instrumented arthrodesis) compared with a group of patients treated in another way (e.g., unsintrumented arthrodesis) at the same institution.
fThe study was started after the first patient enrolled.
gPatients identified for the study based on their outcome, called “cases” (e.g., pseudoarthrosis) are compared to those who did not have outcome, called “controls” (e.g., successful fusion).
hPatients treated one way with no comparison group of patients treated in another way.
Appendix IV. Linking Levels of Evidence to Grades of Recommendation
|
Grade of recommendation
|
Standard language
|
Levels of evidence
|
|
A
|
Recommended
|
Two or more consistent level I studies
|
|
B
|
Suggested
|
One level I study with additional supporting level II or III studies
|
Two or more consistent level II or III studies
|
|
C
|
Is an option
|
One level I, II, or III study with supporting level IV studies
|
Two or more consistent level IV studies
|
|
Insufficient
(insufficient or conflicting evidence)
|
Insufficient evidence to make recommendation for or against
|
A single level I, II, III, or IV study without other supporting evidence
|
>1 study with inconsistent findingsa
|
aNote that in the presence of multiple consistent studies, and a single outlying, inconsistent study, the Grade of Recommendation will be based on the level of the consistent studies.
Appendix V. Criteria Grading the Evidence
The task force used the criteria provided below to identify the strengths and weaknesses of the studies included in this guideline. Studies containing deficiencies were downgraded one level (no further downgrading allowed, unless so severe that study had to be excluded). Studies with no deficiencies based on study design and contained clinical information that dramatically altered current medical perceptions of topic were upgraded.
- Baseline study design (i.e. therapeutic, diagnostic, prognostic) determined to assign initial level of evidence.
- Therapeutic studies reviewed for following deficiencies:
- Failure to provide a power calculation for an RCT;
- High degree of variance or heterogeneity in patient populations with respect to presenting diagnosis/demographics or treatments applied;
- Less than 80% of patient follow-up;
- Failure to utilize validated outcomes instrument;
- No statistical analysis of results;
- Cross over rate between treatment groups of greater than 20%;
- Inadequate reporting of baseline demographic data;
- Small patient cohorts (relative to observed effects);
- Failure to describe method of randomization;
- Failure to provide flowchart following patients through course of study (RCT);
- Failure to account for patients lost to follow-up;
- Lack of independent post-treatment assessment (e.g., clinical, fusion status, etc.);
- Utilization of inferior control group:
- Historical controls;
- Simultaneous application of intervention and control within same patient.
- Failure to standardize surgical/intervention technique;
- Inadequate radiographic technique to determine fusion status (e.g. – static radiographs for instrumented fusion).
- If an RCT fails criteria specific to RCT (such as method randomization reported or improper, no power, greater that 20% crossover, if there is or is not post treatment assessment, inappropriate statistics, no baseline data, small cohorts, etc.), then it will be initially assigned to level II. Only if it further fails additional evaluation, can it be downgraded further to a level III.
- Methodology of diagnostic studies reviewed for following deficiencies:
- Failure to determine specificity and sensitivity;
- Failure to determine inter- and intra-observer reliability;
- Failure to provide correlation coefficient in the form of kappa values.
- Methodology of prognostic studies reviewed for following deficiencies:
- High degree of variance or heterogeneity in patient populations with respect to presenting diagnosis/demographics or treatments applied;
- Failure to appropriately define and assess independent and dependent variables (e.g., failure to use validated outcome measures when available).
© Congress of Neurological Surgeons
Source: Neurosurgery, September 6, 2018