Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients With Painful Diabetic Neuropathy A Randomized Clinical Trial
- Diabetic peripheral neuropathy and chronic pain Methods
- Randomized, controlled trial of 10-kHz spinal cord stimulation
- Prospective, multicenter, open-label
- Spinal cord stimulation versus conventional medical management
- SCS details
- percutaneous leads, T8-T11 placement, 5-7 day trial period
- 10-kHz frequency, 30 microsecond pulse width, amplitude 0.5-3.5 mA
- Inclusion
- Pain for at least 1 year
- Refractory to gabapentin and at least 1 other medication
- Pain intensity 5/10 or more on VAS
- BMI < 45
- Hemoglobin A1C < 10%
- Daily morphine equivalents <120mg
- 6 month follow up, optional crossover at 6 months
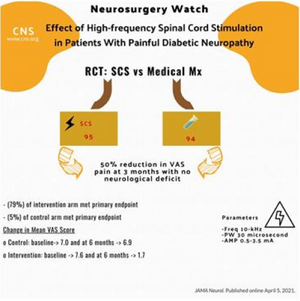
- Primary endpoint: 50% reduction in VAS pain at 3 months with no neurological deficit
- 430 screened: 214 excluded or declined, 216 randomized, 187 evaluated
Results
- 60% male; Mean age 60.8 (SD 10.7)
- Intention-to-treat analysis
- Primary endpoint met
- 5/94 (5%) of control arm
- 75/95 (79%) of intervention arm
- Difference between control and intervention: 73.6%, 95% CI 64-83, p<0.001)
- Mean VAS Score
- Control 7.0 baseline à9 at 6 months
- Intervention 7.6 baseline à7 at 6 months
- Quality of life as secondary endpoint (EQ-5D): significant improvement in intervention group
Limitations
- No blinding
- Possible placebo effect
Source
JAMA Network