- This updated guideline is the result of a planned five-year review of medical literature conducted by the Congress of Neurological Surgeons. This update discusses new and previous recommendations from the 2014 guidelines on the treatment of pediatric hydrocephalus (HC).
- There are 1 new Level III and 1 new Level I recommendations while the rest of the guidelines remain unchanged. These include:
Level III – Management of post hemorrhagic HC in premature infants:
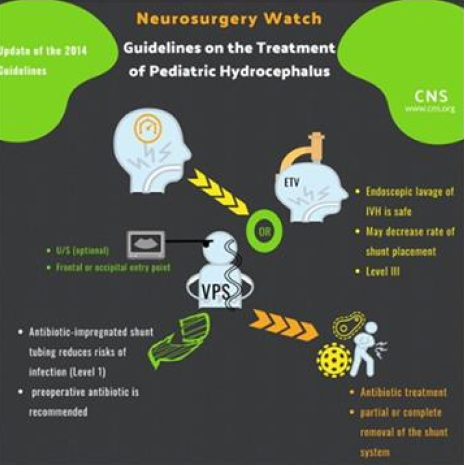
- NEW: Neuro-endoscopic lavage is a feasible and safe option for removal of intraventricular clots and may decrease rate of shunt placement.
Level I - Antibiotic-impregnated shunt systems vs conventional shunts to prevent shunt infection in children:
- NEW: Antibiotic-impregnated shunt tubing reduces risks of infection compared to the conventional silicone hardware and should be used for children who require shunt placement
Confirmation of previous recommendations:
- Technical assistance devices for ventricular placement: ultrasound is an option
- Ventriculoperitoneal shunt (VPS) placement vs endoscopic third ventriculostomy (ETV) for pediatric HC: VPS and ETV are both viable options
- Different shunt components for pediatric VPS: Valve types do not influence risk of shunt failure
- Preoperative antibiotics for VPS: Use of preoperative antibiotic is recommended
- Treatment of VPS infection: Supplementation of antibiotic treatment with partial or complete shunt hardware removal is an option
- Ventricular catheter entry point on shunt survival: frontal and occipital entry points are both options
- Change in ventricle size as a measurement of effective treatment of HC: insufficient evidence to recommend specific change in ventricle size as a measurement of timing and success of HC treatments including VPS and ETV
Source
Academic OUP