Guidelines on Subthalamic Nucleus and Globus Pallidus Internus Deep Brain Stimulation for the Treatment of Patients with Parkinson’s Disease
Internally Produced & Endorsed
Sponsor: Congress of Neurological Surgeons (CNS) and the American Society for Stereotactic and Functional Neurosurgery
Section: Stereotactic & Functional
Download PDF Neurosurgery, 2018
Endorsed by:
Joint Guidelines Committee of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS)
Anand Rughani, MD1, Jason M. Schwalb, MD2, Christos Sidiropoulos, MD3, Julie Pilitsis, MD, PhD4, Adolfo Ramirez-Zamora, MD5, Jennifer A. Sweet, MD6, Sandeep Mittal, MD7, Alberto J. Espay, MD, MSc8, Jorge Gonzalez Martinez, MD, PhD9, Aviva Abosch, MD, PhD10, Emad Eskandar, MD11, Robert Gross, MD, PhD12, Ron Alterman, MD13, Clement Hamani, MD, PhD14
- Neuroscience Institute, Maine Medical Center, Portland, Maine, USA
- Department of Neurosurgery, Henry Ford Medical Group, West Bloomfield, Michigan, USA
- Department of Neurology and Ophthalmology, Michigan State University, Michigan, USA
- Departments of Neuroscience and Experimental Therapeutics and of Neurosurgery, Albany Medical College, Albany, New York, USA
- Center for Movement Disorders and Neurorestoration, Gainesville, Florida, USA
- Department of Neurosurgery, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA
- Department of Neurosurgery, Wayne State University, Detroit, Michigan, USA
- James J and Joan A Gardner Center for Parkinson Disease and Movement Disorders, University of Cincinnati, Cincinnati, Ohio, USA
- Neurological Institute, Cleveland Clinic, Cleveland, Ohio, USA
- Department of Neurosurgery, University of Colorado School of Medicine, Aurora, Colorado, USA
- Department of Neurological Surgery, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA
- Department of Neurosurgery, Emory University, Atlanta, Georgia, USA
- Division of Neurosurgery Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts, USA
- Division of Neurosurgery, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada
Correspondence:
Clement Hamani, MD, PhD
Division of Neurosurgery
Sunnybrook Health Sciences Centre
2075 Bayview av, Toronto, ON M4N3M5
Canada
Phone: 416 4802100
E-mail: clement.hamani@sunnybrook.ca
Keywords: Deep brain stimulation, globus pallidus internus, guidelines, neuromodulation; Parkinson’s disease, subthalamic nucleus
Abbreviations
ALDS: Academic Medical Center Linear Disability Scale
CDRS: Clinical dyskinesia rating scale
DBS: Deep brain stimulation
GPi: Globus pallidus internus
PD: Parkinson’s disease
PDQ-39: Parkinson’s Disease Questionnaire
SIP: Sickness Impact Profile
STN: Subthalamic nucleus
UPDRS: Unified Parkinson's Disease Rating Scale
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a physician volunteer task force as an educational tool that reflects the current state of knowledge at the time of completion. Each chapter is designed to provide an accurate review of the subject matter covered. This guideline is disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
ABSTRACT
Background: Deep brain stimulation (DBS) is an established therapy for improving motor symptoms and levodopa-induced dyskinesias in patients with Parkinson’s disease (PD). Two different targets are FDA-approved for the treatment of Parkinson’s disease: the subthalamic nucleus (STN) and globus pallidus internus (GPi). While there is evidence to suggest that both are effective in treating motor symptoms when compared to best medical management alone, circumstances in which 1 target should be selected over the other are still debated. The authors systematically reviewed the literature and classified the quality of evidence for the use of STN and GPi DBS in patients with PD.
Methods: A systematic literature search was performed using the PubMed database, searching for articles published between 1966 and February 2017 by combining 2 different strategies. The first was performed for “globus pallidus” and “subthalamic nucleus,” limited to clinical trials in human subjects published in English. The second was for “pallidal” and “subthalamic nucleus” and “deep brain stimulation” limited to English language and human subjects. Abstracts of the combined results were reviewed. A total of 18 clinical series containing at least 10 patients with Parkinson’s disease treated with bilateral STN or GPi DBS for a minimum of 6 months were retrieved for full-text review and analysis. The quality of the articles was assigned to each study and the strength of recommendation classified according to the guidelines development methodology of the AANS/CNS Joint Guidelines Committee.
Results: Based on articles providing Class I data, the following could be derived: bilateral STN DBS is at least equivalent to bilateral GPi DBS in treating motor symptoms, with conflicting evidence regarding possible superiority of STN DBS in some conditions. Bilateral STN DBS is more effective than bilateral GPi DBS in allowing reduction of dopaminergic medications. Bilateral STN and GPi DBS are equally effective in treating medication-induced dyskinesias. Bilateral STN and GPi DBS are equally effective in improving quality of life. Bilateral STN DBS is associated with greater impact on neurocognitive decline on specific domains compared to bilateral GPi DBS. Bilateral STN DBS is associated with a higher or equal risk of mood disturbance than GPi DBS in Parkinson Disease.
Conclusion: Based on evidence, the following recommendations can be made:
- Given that bilateral STN DBS is at least as effective as bilateral GPi DBS in treating motor symptoms of Parkinson’s disease (as measured by improvements in UPDRS-III scores) consideration can be given to the selection of either target in patients undergoing surgery to treat motor symptoms (Level I).
- When the main goal of surgery is reduction of dopaminergic medications in a patient with Parkinson’s disease, then bilateral STN DBS should be performed instead of GPi DBS (Level I).
- There is insufficient evidence to make a generalizable recommendation regarding the target selection for reduction of dyskinesias. However, when the reduction of medication is not anticipated and there is a goal to reduce the severity of ‘on’ medication dyskinesias, the GPi should be targeted (Level I).
- When considering improvements in quality of life in a patient undergoing DBS for Parkinson’s disease, there is no basis to recommend bilateral DBS in 1 target over the other (Level I).
- If there is significant concern about cognitive decline, particularly in regards to processing speed and working memory in a patient undergoing DBS, then the clinician should consider using GPi DBS rather than STN DBS while taking into consideration other goals of surgery (Level I).
- If there is significant concern about the risk of depression in a patient undergoing DBS, then the clinician should consider using pallidal rather than STN stimulation while taking into consideration other goals of surgery (Level I).
- There is insufficient evidence to recommend bilateral DBS in 1 target over the other in order to minimize the risk of surgical adverse events.
INTRODUCTION
The efficacy of bilateral deep brain stimulation (DBS) for the treatment of motor symptoms and levodopa-induced dyskinesias in Parkinson’s Disease (PD) patients is well established.1-7 However, the effectiveness of selecting different stimulation targets is less clear. To date, 2 different targets have been proposed for the treatment of motor symptoms of PD, the subthalamic nucleus (STN) and globus pallidus internus (GPi). While there is evidence to suggest that both are effective when combined with best medical treatment versus best medical treatment alone,6 the circumstances in which one target should be selected over the other are still disputed. It is also unknown whether STN and GPi DBS induce similar benefits. Previously published guidelines suggested that STN stimulation improved motor function, and reduced “off” time, dyskinesias, and medication usage.1 When that guideline was published, however, there was insufficient evidence to support or refute the efficacy of GPi DBS in advanced PD. Since then, a growing number of studies have shown that bilateral GPi DBS is also effective for the treatment of motor symptoms.
In this guideline, the authors systematically review the literature and grade the quality of evidence for the use of STN and GPi DBS in patients with PD using the established methodology endorsed by the Congress of Neurological Surgeons (CNS) and the American Association of Neurological Surgeons (AANS), which can be viewed at https://www.cns.org/guidelines/guideline-development-methodology.
METHODS
Questions Addressed in the Guideline
- Is bilateral STN DBS more, less, or as effective as bilateral GPi DBS in treating motor symptoms of PD, as measured by improvements in Unified Parkinson's Disease Rating Scale, part III (UPDRS-III) scores?
- Is bilateral STN DBS more, less, or as effective as bilateral GPi DBS in allowing reduction of dopaminergic medication in PD?
- Is bilateral STN DBS more, less, or as effective as bilateral GPi DBS in treating dyskinesias associated with PD?
- Is bilateral STN DBS more, less, or as effective as bilateral GPi DBS in improving quality of life measures in PD?
- Is bilateral STN DBS associated with greater, lesser, or a similar impact on neurocognitive function than bilateral GPi DBS in PD?
- Is bilateral STN DBS associated with a higher, lower, or similar risk of mood disturbance than GPi DBS in PD?
- Is bilateral STN DBS associated with a higher, lower, or similar risk of adverse events compared to GPi DBS in PD?
Search Strategy
A systematic review was performed in accordance with PRISMA guidelines.8 A PubMed search was conducted for articles published between 1966 and February 2017 by the first author and replicated by the senior author. The PubMed was used as a single search engine as it allows access to MEDLINE, a commonly used database of references and abstracts on life sciences and biomedical topics worldwide. Two different search strategies were used and the results were combined. The first search was performed for “globus pallidus” and “subthalamic nucleus,” limited to clinical trials in human subjects published in English. The second search was for “pallidal” and “subthalamic nucleus” and “deep brain stimulation” and was limited to English language and human subjects. The first search strategy yielded 87 results, and the second 81 results. Of these 168 results, 17 were found in common to both search strategies, yielding 151 unique abstracts. The first and senior author reviewed abstracts of combined, non-duplicated results. Studies were selected on the basis of the inclusion and exclusion criteria described below. Primary articles were distributed for review by 2 independent reviewers, in addition to either the first or senior author. The independent reviewers were comprised of 3 fellowship-trained attending Movement Disorders Neurologists and 4 fellowship-trained attending Functional Neurosurgeons.
Inclusion Criteria
Published clinical series containing at least 10 PD patients treated with bilateral DBS delivered to the STN or GPi with a minimum follow-up of 6 months. These cutoffs were selected because smaller cohorts would have yielded less than 5 individuals per target and 6 to 12 months are common timelines selected for data reporting in the PD literature.
Inclusion Criteria
Studies were excluded from analysis if they offered only case reports, pre-clinical data, letters to the editor, reviews, or meta-analyses. Studies were also excluded if they included patients who had only unilateral DBS, multiple DBS targets, lesions, had follow-up of less than 6 months in duration, had fewer than 10 patients, included patients with an indication other than PD, were principally concerned with surgical technique, electrophysiology, or neuroimaging, or did not directly make a comparison of clinical outcomes between the 2 targets. Unilateral DBS is not uncommonly performed in clinical practice, but is often performed for a more heterogeneous population of patients than is bilateral DBS. For example, a common indication for unilateral STN DBS is a patient with tremor-dominant PD without other cardinal features. Alternatively, unilateral GPi DBS may be considered in a case of severe contralateral painful dystonia. In order to most appropriately address the questions posed, unilateral DBS was excluded. Papers were also excluded if they published redundant data from the same center. A single paper could meet several exclusion criteria.
Strength of Evidence
For each of the studies included in the analysis, evidence classification and strength of recommendations were graded according to the AANS/CNS criteria (Table 1). The class of evidence (ie, Class I, II, or III) assigned to each article was based on study design, data analysis, and follow-up. Where there was discrepancy between the classes of evidence assigned by 2 primary reviewers, the senior author adjudicated the decision. The strength of recommendation (ie, Level I, II, or III) was linked to the level of evidence supporting that specific recommendation. For each of the included studies, the authors discussed the limitations. Where the original paper did not calculate percentage increases or decreases, this was calculated by the authors. Where the original papers reported both a median and a mean, calculations were performed with the mean, as this was more systematically reported. If the authors reported that a specific comparison (between GPi and STN) was not significant (NS), this was conveyed in the tables. Unreported statistical results are noted as non-reported (NR).
Evaluation of Motor Improvement
Preoperatively, UPDRS-III scores are often obtained to assess the severity of motor symptoms in patients given dopaminergic medication (“medication on”) or with the medication withheld (“medication off”). After DBS implantation, the UPDRS-III score can be measured with or without dopaminergic medications (“on/off” medication) or DBS (“on/off” stimulation). The percentage improvement in the score from baseline allows the 2 treatment groups to be compared. These data points were collected from each study.
Evaluation of Dyskinesias
Reduction in dyskinesias can be measured with both patient diaries and clinical observation. Hours per day of bothersome dyskinesias can be recorded by patients in a diary. Severity of dyskinesias is often measured in a clinical setting with various rating scales, one example being the CDRS (clinical dyskinesia rating scale, 0-28). Similarly, items 32 and 33 of the UPDRS, part IV can capture the duration and disability of dyskinesias.9
Adverse Events
There was wide variation in reporting and categorization of adverse events. The authors have largely considered studies in which these events were systematically described.
RESULTS
The combined search queries yielded a total of 151 unique abstracts (Figure 1). The authors eliminated: i) 12 papers that included patients who underwent surgery aimed at multiple targets or had unilateral DBS in their cohorts; ii) 34 papers that were concerned primarily with surgical technique, electrophysiology, neuroimaging, or functional neuroimaging; iii) 19 papers that included or focused on disease entities other than PD; iv) 5 papers with inadequate follow-up or sample size; v) 23 papers that were review articles, case reports, editorials, or were considered highly redundant, yielding no new outcome data; and vi) 40 studies that did not offer clinical outcome data comparing GPi and STN DBS.
Independent graders reviewed each unique article according to AANS/CNS criteria (Table 1). A total of 18 articles were included in the final analysis (summarized in Tables 2 and 3). Of these publications, several contained long-term follow-up data or were sub-analyses of the same cohort of patients, such as the NSTAPS Study Group10, 11 and the Veterans Affairs (VA) Cooperative Studies Program 468 study group.12, 13 In these instances, the strength of evidence was evaluated independently of the prior publication.
Question 1: Is bilateral STN DBS more, less, or as effective as bilateral GPi DBS in treating motor symptoms of PD, as measured by improvements in Unified Parkinson's Disease Rating Scale, part III (UPDRS-III) scores?
Class I evidence is provided by 3 studies (Tables 4 and 5).11,12,14 In a large randomized controlled double-blinded study, Odekerken et al11 demonstrated that at 1 year postoperatively there was a significant improvement in “off” medication “on” stimulation UPDRS-III scores from baseline in STN DBS-treated patients compared to those receiving stimulation in the GPi. This finding was sustained after 3-year follow-up.15 This study also showed that STN and GPi DBS-treated patients had a similar improvement in UPDRS-III scores when assessed “on” medication “on” DBS. In a smaller Class I study, Anderson et al14 demonstrated no difference in improvement between patients receiving STN or GPi DBS at 1 year (off/on or on/on medications/stimulation).
In the VA cooperative study, Follett et al12 showed no difference in motor improvement with either STN or GPi DBS at 2 years after surgery. Three-year follow-up of the same cohort continued to show a similar outcome in these 2 groups.13 Classification of the strength of this data was challenging, and ultimately the 2-year follow-up was regarded as Class I,12 whereas the 3-year follow-up was regarded as Class II.13 In the 2-year study,12 the authors reported that both subjects and assessors were blinded to target, whereas in the 3-year follow-up study,13 there was potential for unblinding of the assessors (personal communication).
A Class III retrospective unmatched study (n = 13 patients) did find that motor scores at 6 months were significantly more improved in the STN group than in the GPi group in the “off” medication “on” stimulation condition.9 This difference, however, diminished when patients were assessed “on” medication/“on” stimulation. A similar finding was produced by another small retrospective Class III study, which demonstrated a 56.6% improvement in the STN group and a 41.7% improvement in the GPi group at 12 months when measured off medication/on stimulation. While the authors concluded that STN stimulation was superior, they did not demonstrate any statistically significant difference between the 2 groups.16 These findings stand in contrast to an additional 6 Class II and III studies that did not demonstrate a difference between the STN and GPi groups at time points ranging from 6 to 60 months.17-22
In summary, 2 Class I,12,14 2 Class II,13,17 and 6 Class III16,18-22 studies found no differences between the 2 targets in motor score improvements at various time points up to 5 years postoperatively in various medication and stimulation conditions. In contrast, 2 studies, including 1 Class I11 study, found that STN stimulation is associated with greater improvement in motor scores assessed in the “off” medication/“on” stimulation condition. The advantage seen in the STN cohort in this study persisted at 3-year follow-up.15 No study to date has demonstrated a difference in the motor response to STN or GPi DBS in the “on” medication/”on” stimulation state.
Question 2: Is bilateral STN DBS more, less, or as effective as bilateral GPi DBS in allowing reduction of dopaminergic medication in PD?
Two Class I studies,11,12 2 Class II studies,13,15 and 4 Class III studies18,20-22 are all concordant in demonstrating a significantly greater reduction in dopaminergic medications following STN stimulation when compared to GPi stimulation (Table 6). One Class I study demonstrated a non-significant trend towards greater medication reduction in the STN group.14 One additional Class III study showed a reduction in dopaminergic medication without reporting on the significance of the difference (greater in the STN group).17 One Class III study showed no significant difference in medication reduction at 6 months in a retrospective review of 24 patients.19
In summary, compelling evidence was derived from 3 Class I studies, 2 Class II studies and 6 Class III studies showing greater reduction in dopaminergic medications following STN than GPi DBS.
Question 3: Is bilateral STN DBS more, less, or as effective as bilateral GPi DBS in treating dyskinesias associated with PD?
As summarized in Table 7, there is Class I evidence from a single study11 that levodopa-induced dyskinesias are reduced to a significantly greater extent with pallidal stimulation than subthalamic stimulation. At 12-month follow-up, the NSTAPS study revealed that on-medication dyskinesias were reduced by 57% from baseline with pallidal stimulation, compared to 21% with STN stimulation, as measured by the clinical dyskinesia rating scale with blinded assessors (P = .01). While this finding suggests that the severity of dyskinesias is reduced in the clinical setting, self-reported 3-day diaries revealed that both treatment groups experienced similar reductions in the time spent with dyskinesias per day. Patients with pallidal stimulation reported 2 additional hours per day in “on” phase without dyskinesias versus 2.1 additional hours with STN stimulation (P = .85). The improvement seen in the GPi group in the NSTAPS study was maintained at 3-year follow-up.15
Two additional Class I studies revealed a non-significant association with improvement of dyskinesias following pallidal stimulation.12, 14 An early randomized controlled trial showed that pallidal stimulation improved baseline dyskinesias by 89% compared to subthalamic stimulation (62%; P = .27) when blinded clinical assessments were performed.14 In the VA CSP study, the patient diaries demonstrate a decrease of 3.2 hours of troublesome dyskinesias per day with GPi stimulation, and a reduction of 2.6 hours per day with STN stimulation at 24 months (P = .20),12 with similar findings being reported at 36 months. At 3-years follow-up, there was similar improvement in dyskinesias observed in both groups in the VA study.13 An additional 8 Class III studies showed no significant benefit of one DBS target over the other with respect to in reduction in dyskinesias.16-18, 20-24
In summary, a single study provides Class I evidence that the severity of “on” medication dyskinesias, but not the amount of time with dyskinesias, is reduced to a greater extent following pallidal stimulation than subthalamic stimulation. The remaining 2 Class I studies showed no significant differences in the reduction of dyskinesias between these surgical targets.
Question 4: Is bilateral STN DBS more, less, or as effective as bilateral GPi DBS in improving quality of life measures in PD?
No study demonstrated a significant difference between the 2 targets regarding improvement in quality of life (Table 8). Class I evidence from 3 studies have shown comparable improvements in quality of life as measured by the UPDRS-II at 1 year,14 the composite Parkinson’s Disease Questionnaire (PDQ-39) at 2 years,12 or a quality of life questionnaire at 1 year.11 With these instruments, improvement in quality of life compared to baseline ranged from 9% to 28% with no statistically significant difference between GPi and STN groups. Three-year follow-up in the VA study utilized the PDQ-39 and did not reveal a difference between the 2 surgical targets, as shown by a single Class II study.13 Six Class III studies showed improvements in quality of life following DBS without differences between the 2 targets. Studies showed that there was no difference in improvement on UPDRS-II scores at 6 months,18 12 months,20,23 36 months,21 or 60 months.17 At 36 months, no significant difference was observed between the 2 targets with the Sickness Impact Profile (SIP).24 Only 1 of these studies addressed different subdomains,13 showing improvements in mobility, activities of daily living, emotional role function, stigma, cognition, communication, and body discomfort. There were no statistically significant differences between surgical targets in these subdomains.
Question 5: Is bilateral STN DBS associated with greater, lesser or a similar impact on neurocognitive function than bilateral GPi DBS in PD?
Neurocognitive function was formally assessed using various batteries in 5 studies.10-13, 25 Class I evidence was provided by 3 of these studies, in which patients and assessors were blinded to the stimulation site.10-12 At 12 months postoperatively, patients undergoing pallidal stimulation experienced a 27% decrease in the cognitive component of the Academic Medical Center Linear Disability Scale (ALDS) as compared to 35% in the STN group.11 A more detailed neurocognitive battery testing attention, working memory, executive function, semantic fluency, language, memory, and spatial reasoning was performed in the same cohort at 12 months.10 Of these domains, there was a greater decline identified in the STN cohort than the GPi cohort with only some of the tasks of mental speed (Stroop word reading and Stroop color naming), attention (trail making test, part B), and possibly language (Wechsler Adult Intelligence Scale similarities).
This finding of a small but significant decline in some cognitive domains in patients who underwent STN DBS is consistent with similar findings from Follett et al12 who demonstrated a significantly greater decline in processing speed and working memory associated with STN DBS compared to GPi DBS at 24 months postoperatively (P = .03). In the 3-year follow-up of this cohort, blinded neurocognitive assessments revealed that STN DBS was associated with statistically significant declines in the Mattis Dementia Rating Scale and the Hopkins Verbal Learning Test, whereas this decline was not observed in the GPi group.13
In a study providing Class III evidence, GPi treated patients had longer response latency in reaction time in 1 study compared to those receiving STN DBS.25
In summary, there is Class I evidence suggesting a greater decline on certain neurocognitive tasks following STN DBS as compared to GPi DBS.
Question 6: Is bilateral STN DBS associated with a higher, lower, or similar risk of mood disturbance than GPi DBS in PD?
Class I evidence from the VA Cooperative Study demonstrated a slight improvement in the GPi group (5.8%) compared to a slight worsening in the STN group (-11.6%) on the Beck Depression Inventory (P = .02).12 There were no differences between groups when this cohort was assessed for suicidal ideation and suicidal behaviors at 6, 12, and 24 months postoperatively using the UPDRS-I.26 When the PDQ-39 was administered at 6 months postoperatively, the study authors noted a greater number of patients report feeling “angry or bitter” in the STN group (P = .004). The authors did not further elaborate on those patients that might be at greater risk of decline in mood or identify other risk factors for decline in mood.
The NSTAPS 3-year follow-up study found that there were no differences between targets when measuring with a composite of mood, cognitive, and behavioral effects (Class II).15
Class III evidence comes from 2 studies. A retrospective study of 27 patients demonstrated that both GPi and STN DBS groups were associated with a trend towards reduced Hamilton Depression Scale scores up to 12 months postoperatively.22 Another retrospective series of 76 patients demonstrated decreases on the Beck Depression Inventory (BDI) score 12 months postoperatively in both the STN DBS group (21.1%) and the GPi group (27.0%).25 Thus, there is Class I evidence from a single study suggesting that GPi stimulation is associated with better outcomes in terms of depression than STN. However, these findings are restricted to a single paper.
Question 7: Is bilateral STN DBS associated with a higher, lower, or similar risk of adverse events compared to GPi DBS in PD?
Table 9 provides a summary of adverse events as reported by individual papers. There is wide variation in methodology and categorization of adverse events. While some studies had systematic questionnaires11,12,27 and some studies further categorized severity, duration, and relationship to the surgery, many studies did not. No study showed a significantly higher risk of adverse events related to one surgical target over another. One notable finding that could be considered an adverse event was seen in the 3-year follow-up data from the NSTAPS study.15 Seventeen percent (8 of 47) of patients in the GPi group underwent subsequent revision surgery due to lack of benefit, whereas only 2.3% (one of 43) of patients in the STN group underwent revision surgery (P = .03).
DISCUSSION
PD is characterized by many symptoms, which present in a variety of combinations and severities, and which have varied responses to both medications and DBS. This clinical heterogeneity can make selection of the appropriate target for DBS somewhat complex. Based on the current literature, there are areas of agreement and disagreement over the question of target superiority in DBS for the treatment of PD. Motor function in the “on” medication “on” stimulation condition seems to improve equally, irrespective of whether STN of GPi is selected as the DBS target. In contrast, there is conflicting Class I data on whether motor symptoms in the “off” medication condition are more effectively controlled by stimulation in the latter target11 or equally improved by GPi or STN DBS.12, 14 On the other hand, there is strong consensus that STN DBS allows a more robust reduction of medications postoperatively. Class I evidence also exists suggesting that GPi may be associated with a greater reduction in dyskinesias. This reduction, however, is not reflected in motor diaries measuring hours per day without dyskinesias. The above-mentioned findings remain compatible with the notion that STN DBS exerts its anti-dyskinetic effect mainly through medication reductions, whereas GPi DBS suppresses dyskinesias directly by mechanisms that remain to be fully elucidated.
In addition to improvements in motor symptoms, DBS delivered to either target is associated with improvements on quality of life scales. Based on Class I evidence from 1 large prospective, randomized trial, some cognitive measures decline more rapidly after STN DBS (eg, processing speed, working memory, and attention) than with GPi. This same study suggested that mood is better with bilateral GPi than STN DBS. Other studies did not corroborate these findings, however, suggesting that STN or GPi DBS equally affect quality of life and cognitive function.
PD has a heterogeneous presentation, and a wide range of symptoms, which can manifest differently across patients. DBS has a valuable role in treating certain motor symptoms. Overall, the best available evidence suggests that bilateral STN and bilateral GPi DBS offer somewhat similar outcomes in terms of motor function (particularly in the “on” medication condition) and quality of life improvements in patients with advanced PD. Both improve levodopa-induced dyskinesias. However, STN offers the opportunity for more substantial dopaminergic medication reduction. Subthalamic nucleus DBS carries an increased risk of neurocognitive deterioration in specific domains and an increased risk of depressed mood than GPi DBS. Ultimately, the selection of a specific brain target for stimulation should be tailored to the needs of the individual patient. In summary, Class I evidence shows:
- Bilateral STN DBS is at least as effective as bilateral GPi DBS in treating the motor symptoms of PD, as measured by improvements in UPDRS-III scores (3 Class I studies show equivalence, and 1 shows an advantage of STN in certain conditions).
- Bilateral STN DBS is more effective than bilateral GPi DBS in allowing reduction of dopaminergic medication in PD (2 Class I studies).
- Bilateral STN and GPi DBS are both effective in treating dyskinesias associated with PD (2 Class I studies demonstrate equivalence in reduction of dyskinesias; 1 Class I study shows an advantage of GPi in reducing severity of ‘on’ medication dyskinesias.)
- Bilateral STN and GPi DBS are equally effective in improving quality of life measures in PD (3 Class I studies demonstrate no differences between the 2 targets).
- Bilateral STN DBS is associated with a greater impact on neurocognitive decline than bilateral GPi DBS on specific domains (3 Class I studies).
- Bilateral STN DBS is associated with a higher risk of mood disturbance than GPi DBS in PD (single Class I study).
- There is insufficient evidence to conclude that there is a higher risk of adverse events following bilateral DBS of the STN versus GPi (no Class I studies).
Although there is an abundance of data demonstrating the efficacy of DBS, a few caveats need to be raised. Well-designed studies providing Class I evidence have shown slightly divergent findings regarding treatment efficacy. While a few trials do not suggest significant differences with the use of either STN or GPi DBS, others indicate that patients treated with the former have a better outcome. Such discrepancies are difficult to explain inasmuch as selection criteria, surgical technique, programming, follow-up time, and management are fairly standard across centers that offer the procedure. The only finding that seems to be clear is that GPi DBS is not superior to STN DBS for improving motor symptoms. In 3 Class I studies that examined motor improvements, none demonstrated an advantage of GPi DBS over STN DBS in any condition11, 12, 14. The only condition in which STN DBS was found to show greater benefit than GPi DBS was when the patients were re-examined off medication with the DBS on at 1 year following surgery. In this condition, there was a greater improvement from baseline in the STN group11. This, however, is a condition, which may not be an accurate indication of what occurs in a non-clinical setting.
It is also difficult to provide conclusive remarks about side effects, mood, and cognition. Overall, there seems to be a common sentiment amongst clinicians that STN DBS is associated with a higher incidence of difficulties with speech processing, and tendencies towards more psychiatric and cognitive side effects as compared to GPi DBS. The studies included here corroborate this sentiment but only insofar as very specific domains of cognitive functioning and mood demonstrated significant differences. One possible reason to account for this discrepancy is the relatively low incidence of side effects, particularly in trials where patients are well screened for psychiatric and cognitive co-morbidity prior to surgery. A large study population would be required to yield statistically significant results. The complexity of cognition and emotional well-being further underscore the difficulty of detecting systematic differences across this population of patients using any one battery of tests. Another complexity with this population is that there can be rapid fluctuations in cognitive performance that occur at a timescale that does not enable completion of testing batteries in a consistent state of performance. That is, the duration of these tests can often exceed the time that the patient has good functioning. Perhaps a more practical approach for future work comparing side effects between targets would be to create repositories of prospectively collected data generated in multiple centers. Evidence to suggest that current studies might be underpowered to detect significant changes between targets stems from the fact that, when looking at absolute values in the tables provided in different studies, the number of patients presenting mood and cognitive symptoms or speech difficulty is often higher following STN DBS. A third aspect that needs to be discussed is related to measurements of outcome in the “on” vs “off” medication conditions. While studies comparing STN vs GPi DBS were often interested in scores obtained without the use of medications, from a patient’s perspective, the benefits obtained with all treatments combined are likely more important.
After discussing these limitations, the authors believe that, based on the currently published studies appraised in this review, the following recommendations can be made:
- Given that bilateral STN DBS is at least as effective as bilateral GPi DBS in treating motor symptoms of PD, as measured by improvements in UPDRS part III scores, consideration can be given to selection of either target in patients undergoing surgery to treat motor symptoms. (Level I).
- When the main goal of surgery is reduction of dopaminergic medications in a patient with PD, then bilateral STN DBS should be performed instead of GPi DBS (Level I).
- There is insufficient evidence to make a generalizable recommendation regarding the target selection for reduction of dyskinesias. However, when the reduction of medication is not anticipated, and there is a goal to reduce the severity of ‘on’ medication dyskinesias GPi should be targeted (Level I).
- When considering improvements in quality of life in a patient undergoing DBS for PD, there is no basis to recommend bilateral DBS in one target over the other (Level I).
- If there is significant concern about cognitive decline, particularly in regards to processing speed and working memory in a patient undergoing DBS, then the clinician should consider using GPi stimulation rather than STN stimulation while taking into consideration other goals of surgery (Level I).
- If there is significant concern about the risk of depression in a patient undergoing DBS, then the clinician should consider using GPi rather than STN stimulation while taking into consideration other goals of surgery (Level I).
- There is insufficient evidence to recommend bilateral DBS in one target over the other in order to minimize the risk of adverse surgical events.
Conflict of Interest
The Deep Brain Stimulation for Parkinson’s Disease Task Force members reported all possible conflicts of interest (COIs) before beginning work on the guideline, using the COI disclosure form of the JGC, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of Task Force Members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs (Table 10).
ACKNOWLEDGEMENTS
The guidelines task force would like to acknowledge the CNS Guidelines Committee for their contributions throughout the development of the guideline, the AANS/CNS Joint Guidelines Review Committee for their review, comments, and suggestions throughout peer review, as well as the contributions of Trish Rehring, MPH, CHES, Senior Manager of Clinical Practice Guidelines for the CNS, and Mary Bodach, MLIS, Senior Guidelines Specialist for the CNS. Throughout the review process the reviewers and authors were blinded from one another. At this time the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: John O’Toole, MD; Maya Babu, MD; Andrew P. Carlson, MD; Jamie Van Gompel, MD; D. Ryan Ormond, MD; Mateo Ziu, MD.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the CNS and the American Society for Stereotactic and Functional Neurosurgery of the CNS and the AANS, which received no funding from outside commercial sources to support the development of this document.
REFERENCES
1. Pahwa R, Factor SA, Lyons KE, et al. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. Apr 11 2006;66(7):983-995.
2. Perestelo-Perez L, Rivero-Santana A, Perez-Ramos J, Serrano-Perez P, Panetta J, Hilarion P. Deep brain stimulation in Parkinson's disease: meta-analysis of randomized controlled trials. Journal of neurology. Nov 2014;261(11):2051-2060.
3. Okun MS, Gallo BV, Mandybur G, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson's disease: an open-label randomised controlled trial. The Lancet. Neurology. Feb 2012;11(2):140-149.
4. Rodriguez-Oroz MC, Moro E, Krack P. Long-term outcomes of surgical therapies for Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. Dec 2012;27(14):1718-1728.
5. Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. The New England journal of medicine. Aug 31 2006;355(9):896-908.
6. Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. Jama. Jan 07 2009;301(1):63-73.
7. Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson's disease with early motor complications. The New England journal of medicine. Feb 14 2013;368(7):610-622.
8. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. Oct 2009;62(10):e1-34.
9. Krack P, Pollak P, Limousin P, et al. Subthalamic nucleus or internal pallidal stimulation in young onset Parkinson's disease. Brain : a journal of neurology. Mar 1998;121 ( Pt 3):451-457.
10. Odekerken VJ, Boel JA, Geurtsen GJ, et al. Neuropsychological outcome after deep brain stimulation for Parkinson disease. Neurology. Mar 31 2015;84(13):1355-1361.
11. Odekerken VJ, van Laar T, Staal MJ, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): a randomised controlled trial. The Lancet. Neurology. Jan 2013;12(1):37-44.
12. Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. The New England journal of medicine. Jun 3 2010;362(22):2077-2091.
13. Weaver FM, Follett KA, Stern M, et al. Randomized trial of deep brain stimulation for Parkinson disease: thirty-six-month outcomes. Neurology. Jul 3 2012;79(1):55-65.
14. Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Archives of neurology. Apr 2005;62(4):554-560.
15. Odekerken VJ, Boel JA, Schmand BA, et al. GPi vs STN deep brain stimulation for Parkinson disease: Three-year follow-up. Neurology. Feb 23 2016;86(8):755-761.
16. Scotto di Luzio AE, Ammannati F, Marini P, Sorbi S, Mennonna P. Which target for DBS in Parkinson's disease? Subthalamic nucleus versus globus pallidus internus. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. Feb 2001;22(1):87-88.
17. Moro E, Lozano AM, Pollak P, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. Apr 15 2010;25(5):578-586.
18. Deep-Brain Stimulation for Parkinson's Disease Study G. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. The New England journal of medicine. Sep 27 2001;345(13):956-963.
19. Evidente VG, Premkumar AP, Adler CH, Caviness JN, Driver-Dunckley E, Lyons MK. Medication dose reductions after pallidal versus subthalamic stimulation in patients with Parkinson's disease. Acta neurologica Scandinavica. Sep 2011;124(3):211-214.
20. Minguez-Castellanos A, Escamilla-Sevilla F, Katati MJ, et al. Different patterns of medication change after subthalamic or pallidal stimulation for Parkinson's disease: target related effect or selection bias? Journal of neurology, neurosurgery, and psychiatry. Jan 2005;76(1):34-39.
21. Rodriguez-Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain : a journal of neurology. Oct 2005;128(Pt 10):2240-2249.
22. Volkmann J, Allert N, Voges J, Weiss PH, Freund HJ, Sturm V. Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology. Feb 27 2001;56(4):548-551.
23. Krause M, Fogel W, Heck A, et al. Deep brain stimulation for the treatment of Parkinson's disease: subthalamic nucleus versus globus pallidus internus. Journal of neurology, neurosurgery, and psychiatry. Apr 2001;70(4):464-470.
24. Volkmann J, Albanese A, Kulisevsky J, et al. Long-term effects of pallidal or subthalamic deep brain stimulation on quality of life in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. Jun 15 2009;24(8):1154-1161.
25. Pillon B, Ardouin C, Damier P, et al. Neuropsychological changes between "off" and "on" STN or GPi stimulation in Parkinson's disease. Neurology. Aug 8 2000;55(3):411-418.
26. Weintraub D, Duda JE, Carlson K, et al. Suicide ideation and behaviours after STN and GPi DBS surgery for Parkinson's disease: results from a randomised, controlled trial. Journal of neurology, neurosurgery, and psychiatry. Oct 2013;84(10):1113-1118.
27. Hariz MI, Rehncrona S, Quinn NP, Speelman JD, Wensing C, Multicentre Advanced Parkinson's Disease Deep Brain Stimulation G. Multicenter study on deep brain stimulation in Parkinson's disease: an independent assessment of reported adverse events at 4 years. Movement disorders : official journal of the Movement Disorder Society. Feb 15 2008;23(3):416-421.
Figure 1. PRISMA Flowchart
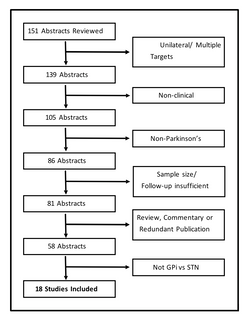
Table 1. AANS/CNS Classification of Evidence and Levels of Recommendation for Therapeutic Effectiveness
| Evidence Classification |
Levels of Recommendation |
| Class I: Evidence provided by at least 1 well-designed, randomized, controlled clinical trial, including meta-analyses of such trials |
Level I: Generally accepted principles for patient management that reflect a high degree of clinical certainty (usually this requires Level I evidence that directly addresses the clinical questions or overwhelming Level II evidence when circumstances preclude randomized clinical trials) |
| Class II: Evidence provided by well-designed observational studies with concurrent controls (eg, case-control and cohort studies) |
Level II: Recommendations for patient management that reflect clinical certainty (usually this requires Level II evidence or a strong consensus of Level III evidence) |
| Class III: Evidence provided by expert opinion, case series, case reports, and studies with historical controls |
Level III: Other strategies for patient management for which the clinical utility is uncertain (inconclusive or conflicting evidence or opinion) |
Table 2. Study Characteristics
| First Author |
Study Design |
Class of Evidence |
Randomized |
Subjects Blinded to DBS Target |
Assessors Blinded to DBS Target |
Matched Groups |
Subjects |
Follow-up (months) |
Percentage Lost to follow-up |
| Anderson et al14 |
RCT |
I |
Yes |
Yes |
Yes |
Yes[A] |
23 |
12 |
13% |
| DBS for PD Study Group18 |
Prospective |
III |
No |
No[B] |
No |
Yes |
134 |
6 |
5% |
| Evidente et al19 |
Retrospective |
III |
No |
No |
No |
Yes |
24 |
6 |
NR |
| Follett et al12 |
RCT |
I |
Yes |
Yes |
Yes |
Yes[C] |
299 |
24 |
13% |
| Hariz et al27 |
Prospective |
III |
No |
No |
No |
No |
89 |
48 |
22% |
| Krause et al23 |
Prospective |
III |
No |
No |
No |
No |
18 |
12 |
11% |
| Minguez-Castellanos et al20 |
Retrospective |
III |
No |
No |
No |
No |
20 |
12 |
N/A |
| Moro et al17 |
Prospective |
III |
No |
No |
No |
Unclear |
51 |
60 |
51% |
| Odekerken et al10 |
RCT |
I |
Yes |
Yes |
Yes |
Yes |
128 |
12 |
11% |
| Odekerken et al11 |
RCT |
I |
Yes |
Yes |
Yes |
Yes |
128 |
12 |
>4% |
| Rodriguez-Oroz et al21 |
Prospective |
III |
No |
No |
No |
Unclear |
105 |
36 |
22% |
| Pillon et al25 |
Retrospective |
III |
No |
No |
No |
No |
76 |
12 |
N/A |
| Volkmann et al24 |
Prospective |
III |
No |
No |
No |
Unclear |
65 |
36 |
NR |
| Volkmann et al22 |
Retrospective |
III |
No |
No |
No |
Unclear |
27 |
12 |
N/A |
| Weaver et al13 |
RCT – long-term follow-up |
II |
Yes |
Yes |
Yes |
Yes[D] |
159 |
36 |
20% |
| Weintraub et al26 |
RCT – sub-analysis |
III |
Yes |
Yes |
Yes |
Yes |
299 |
24 |
10% |
| Odekerken et al15 |
RCT – long-term follow-up |
III |
Yes |
Yes |
Yes |
Yes |
128 |
36 |
30% |
| Scotto di Luzio et al16 |
Retrospective |
III |
No |
No |
No |
No |
14 |
12 |
NR |
Table 3. Patient Demographics
| First Author |
Age - STN (years) |
Age – GPi (years) |
Duration – STN (years) |
Duration – GPi (years) |
UPDRS-III off meds – STN |
UPDRS-III off meds - GPi |
LEED – STN (mg) |
LEED – GPi (mg) |
| Anderson at al14 |
61 |
54 |
15.6 |
10.3 |
49 |
51 |
NR |
NR |
| DBS for PD Study Group18 |
59 |
55.7 |
14.4 |
14.5 |
54.0 |
50.8 |
1218 |
1090 |
| Evidente at al19 |
66.4 |
66.9 |
NR |
NR |
40.0 |
40.1 |
1106 |
1042 |
| Follett et al12 |
61.9 |
61.8 |
11.1 |
11.5 |
43.0 |
41.8 |
1118 |
1361 |
| Krause et al23 |
58.7 |
58.5 |
13.7 |
14.7 |
NR |
NR |
NR |
NR |
| Minguez-Castellanos et al20 |
62.0 |
59.0 |
14.8 |
15.2 |
58.5 |
63.4 |
1394 |
762 |
| Moro et al17 |
59.6 |
54.4 |
12.6 |
12.6 |
32.5 |
26.8 |
1475 |
1275 |
| Odekerken et al10 |
60.3 |
59.2 |
12.3 |
10.9 |
NR |
NR |
1200 |
1226 |
| Odekerken et al11 |
60.9 |
59.1 |
12.0 |
10.8 |
44.4 |
43.8 |
1254 |
1331 |
| Rodriguez-Oroz et al21 |
59.8 |
55.8 |
15.4 |
15.4 |
56.7 |
51.7 |
1336 |
1074 |
| Pillon et al25 |
55.2 |
53.5 |
14.8 |
14.8 |
55.6 |
50.1 |
1098 |
784 |
| Volkmann et al24 |
58.5 |
55.8 |
15.3 |
15.4 |
NR |
NR |
NR |
NR |
| Volkmann et al22 |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
| Weaver et al13 |
60.7 |
60.4 |
11.3 |
11.4 |
42.5 |
41.1 |
1270 |
1365 |
| Weintraub et al26 |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
| Odekerken et al15 |
60.9 |
59.1 |
12.0 |
10.8 |
41 |
43 |
NR |
NR |
| Scotto di Luzio et al16 |
54.9 |
55.4 |
11.0 |
8.4 |
NR |
NR |
NR |
NR |
| Hariz et al 27 |
59.8 |
55.8 |
15.4 |
15.4 |
56.7 |
51.7 |
1309 |
1074 |
Table 4. Percent Improvement in UPDRS-III from Baseline (“off” medication) to Follow-up (“off” medication/“on” stimulation)
| Reference |
Percent Improvement: STN |
Percent Improvement: GPi |
Significance |
| Class I |
| Anderson et al14 |
39% |
48% |
.40 |
| Follett et al12 |
25.3% |
28.2% |
.50 |
| Odekerken et al11 |
45.7% |
26% |
.03 |
| Class II |
| Moro et al17 |
46.9% |
46.7% |
NR |
| Weaver et al13 |
43.5% |
50.4% |
NR |
| Odekerken et al15 |
33.3% |
23.2% |
.04 |
| Class III |
| DBS for PD Study Group18 |
52.4% |
33.3% |
NR |
| Minguez-Castellanos et al20 |
40.2% |
37.5% |
NS |
| Rodriguez-Oroz et al21 |
56.6% |
44.7% |
NR |
| Volkmann et al22 |
60.3% |
68.2% |
NR |
| Scotto di Luzio et al16 |
56.6% |
41.7% |
NR |
Table 5. Percent Improvement in UPDRS-III from Baseline (“off” medication) to Follow-up (“on” medication/“on” stimulation)
| Reference |
Percent Improvement: STN |
Percent Improvement: GPi |
Significance |
| Class I |
| Anderson et al14 |
64% |
61% |
.83 |
| Follett et al12 |
46% |
48.8% |
NR |
| Odekerken et al11 |
67.6% |
63.5% |
.17 |
| Class II |
| Weaver et al13 |
43.5% |
50.4% |
NR |
| Class III |
| DBS for PD Study Group18 |
67% |
67.5% |
NR |
| Evidente et al19 |
60% |
58% |
.94 |
| Minguez-Castellanos et al20 |
72.4% |
62.6% |
NR |
| Moro et al17 |
59.3% |
62.9% |
NR |
| Rodriguez-Oroz et al21 |
64.2% |
65.8% |
NR |
| Volkmann et al22 |
70.9% |
68.2% |
NR |
Table 6. Reduction in Dopaminergic Medication
| Reference |
Reduction in LEED (%) – STN |
Reduction in LEED (%) – GPi |
Significance |
| Class I |
| Anderson et al14 |
38% |
3% |
.08 |
| Follett et al12 |
31% |
18% |
.02 |
| Odekerken et al11 |
44% |
16% |
.01 |
| Class II |
| Weaver et al13 |
36% |
18% |
<.001 |
| Odekerken15 |
NR |
NR |
<.001 |
| Class III |
| DBS for PD Study Group18 |
37% |
-1% |
<.001 |
| Evidente et al19 |
37% |
48% |
.52 |
| Minguez-Castellanos et al20 |
24% |
-9% |
.02 |
| Moro et al17 |
53% |
16% |
NR |
| Rodriguez-Oroz et al21 |
34% |
32% |
<.001 |
| Volkmann et al22 |
63% |
28% |
<.001 |
Table 7. Reduction in Dyskinesias
| Reference |
Notes |
Improvement in Dyskinesias – STN |
Improvement in Dyskinesias – GPi |
Significance |
| Class I |
| Anderson et al14 |
[E] |
62% |
89% |
.27 |
| Follett et al12 |
[F] |
65% |
73% |
.20 |
| Odekerken et al11 |
[G] |
21% |
57% |
.01 |
| Class II |
| Weaver et al13 |
|
37% |
41% |
NR |
| Odekerken15 |
[H] |
NR |
NR |
.02 |
| Class III |
| DBS for PD Study Group18 |
[I] |
58% |
67% |
NR |
| Krause et al23 |
[J] |
58% |
58% |
NR |
| Minguez-Castellanos et al20 |
[K] |
42% |
56% |
NS |
| Moro et al17 |
|
83% |
75% |
NR |
| Rodriguez-Oroz et al21 |
|
59% |
75% |
NR |
| Volkmann et al24 |
|
58% |
79% |
NR |
| Volkmann et al22 |
|
90% |
83% |
NR |
| Scotto di Luzio et al16 |
|
74% |
55% |
NR |
Table 8. Improvement in Quality of Life
| Reference |
Measure |
Change – STN |
Change – GPi |
Significance |
| Class I |
| Anderson et al14 |
UPDRS-II |
28% |
18% |
.48 |
| Follett et al12 |
PDQ 39 |
9% |
11% |
.69 |
| Class II |
| Weaver et al13 |
PDQ 39 |
9% |
6% |
.38 |
| Class III |
| DBS for PD Study Group18 |
UPDRS-II |
44% |
36% |
NR |
| Krause et al23 |
UPDRS-II |
26% |
5% |
NR |
| Minguez-Castellanos et al20 |
UPDRS-II |
33% |
32% |
NR |
| Moro et al17 |
UPDRS-II |
38% |
26% |
NR |
| Rodriguez-Oroz et al21 |
UPDRS-II |
43% |
28% |
NR |
| Volkmann et al24 |
Sickness Impact Profile |
21% |
18% |
NR |
Table 9. Adverse Events Reported
| First Author |
STN Adverse Events |
GPi Adverse Events |
| Anderson et al14 |
30% |
20% |
| DBS for PD Study Group18 |
37% |
49% |
| Follett et al12 |
51% |
37% |
| Krause et al23 |
42% |
33% |
| Minguez-Castellanos et al20 |
10% |
10% |
| Moro et al17 |
74% |
50% |
| Odekerken et al11 |
303/63 |
290/65 |
| Rodriguez-Oroz et al21 |
53% |
35% |
| Volkmann et al24 |
53% |
35% |
| Odekerkan et al15 |
12/43 |
21/47 |
| Hariz et al27 |
53% |
35% |
Table 10. Conflict of interest disclosures and affiliations
| Guideline Author |
Affiliations |
Potential Conflict(s) of Interest |
| Aviva Abosch, MD, PhD |
Department of Neurosurgery, University of Colorado School of Medicine |
Grant: Alpha Omega Consultant fee: Medtronic (ad hoc) Honorarium: Alpha Omega |
| Ron Alterman, MD |
Division of Neurosurgery Beth Israel Deaconess Medical Center and Harvard Medical School |
No disclosures |
| Emad Eskandar, MD |
Department of Neurological Surgery, Massachusetts General Hospital and Harvard Medical School |
No disclosures |
| Alberto J. Espay, MD, MSc |
James J and Joan A Gardner Center for Parkinson Disease and Movement Disorders, University of Cincinnati |
Grants: NIH, Great Lakes Neurotechnologies, and the Michael J. Fox Foundation Scientific Advisory Boards: Abbvie, TEVA, Impax, Merz, Acadia, Cynapsus/Sunovion, Lundbeck, and USWorldMeds Honoraria: Abbvie, UCB, USWorldMeds, Lundbeck, Acadia, the American Academy of Neurology, and the Movement Disorders Society Royalties: Lippincott, Williams & Wilkins, Cambridge University Press, Springer |
| Jorge Gonzalez-Martinez, MD, PhD |
Neurological Institute, Cleveland Clinic |
No disclosures |
| Robert Gross, MD, PhD |
Department of Neurosurgery, Emory University |
Grants/Research support: Medtronic, Boston Scientific, Neuropace, MRI Interventions, SanBio Consultant fee: Medtronic, Neuropace, MRI Interventions, Neural Stem, SanBio, Monteris Honoraria: Medtronic, Neuropace, MRI Interventions, Zimmer Biomet, Monteris |
| Clement Hamani, MD, PhD |
Division of Neurosurgery, Sunnybrook Health Sciences Centre |
Honoraria: St. Jude Medical and Medtronic |
| Sandeep Mittal, MD |
Department of Neurosurgery, Wayne State University |
Grants/Resarch support: NIH R01CA123451, Novocure, Ltd., Roche Glycart AG, UCB Pharma |
| Julie Pilitsis, MD, PhD |
Departments of Neuroscience and Experimental Therapeutics and of Neurosurgery, Albany Medical College |
Grants/Research support: Medtronic, Boston Scientific, Abbott, Jazz Pharmaceuticals, GE Global Research, St. Jude, NIH 1R01CA166379 Consultant fee: Medtronic, Boston Scientific, Abbott, Centauri, St. Jude Stock shareholder: Centauri Board, trustee or officer position: NANS Scientific Programming Committee Chair, AANS/CNS Pain Section Immediate Past Chair, Co-Director of WIN Other: Medical Advisor, Centauri |
| Adolfo Ramirez-Zamora, MD |
Center for Movement Disorders and Neurorestoration |
Consultant fee: Medtronic and Teva Neuroscience |
| Anand Rughani, MD |
Neuroscience Institute, Maine Medical Center |
No disclosures |
| Jason Schwalb, MD |
Department of Neurosurgery, Henry Ford Medical Group |
Grants/Research support: Medtroic, Inc., Boston Scientific, Inc. |
| Christos Sidiropoulos, MD |
Department of Neurology and Ophthalmology, Michigan State University |
No disclosures |
| Jennifer Sweet, MD |
Department of Neurosurgery, University Hospitals Cleveland Medical Center |
Grants/Research support: KL2, NIH grant, 2KL2TR000440, NIH NCATS Board, Trustee or Officer position: Scientific Advisory Board, Heling Medical Technologies |
[A]Groups were well-matched with the exception of significantly longer disease duration in the STN group.
[B] The patients and assessors were both blinded to the DBS being turned ON or OFF only at the three-month evaluation, but were not blinded to the target location, and were not blinded at the six-month evaluation.
[C] Groups were well-matched in baseline demographics, but did demonstrate differences in PDQ-39 subscores and subscores of verbal fluency and verbal learning at baseline.
[D] See footnote (3) above
[E] Blinded assessment using a 24-point dyskinesia rating scale (0-4 for 6 body areas).
[F] Reported as the change in percentage of time per day (from baseline) with troublesome dyskinesias from self-reporting.
[G] As measured by assessment with the CDRS (score of 0-28) by a blinded evaluator; number here are for ON medication phase.
[H] In the three-year NSTAPS follow-up the paper does not report the percentage improvement for each group, but reports that the CDRS improved by 3.3 in the STN group compared to 2.2 in the GPi group.
[I] Blinded assessment on the dyskinesia-rating scale (0-4).
[J] As measured by UPDRS items 32-35.
[K] As measured by the CAPIT dyskinesia rating scale (0-5).
© Congress of Neurological Surgeons